Volume 12, Issue 2 (Spring 2023)
J Occup Health Epidemiol 2023, 12(2): 114-122 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemi Habybabady R, Mohammadi M, Jafari H, Paridokht F. Protective Effect of N-Acetylcysteine against Continuous Noise-Induced Hearing Loss. J Occup Health Epidemiol 2023; 12 (2) :114-122
URL: http://johe.rums.ac.ir/article-1-675-en.html
URL: http://johe.rums.ac.ir/article-1-675-en.html
Related article in
Google Scholar
Google Scholar
Similar articles
1- Assistant Prof., Health Promotion Research Center, Dept. of Occupational Health Engineering , Zahedan University of Medical Sciences, Zahedan, Iran.
2- Professor in Biostatistics, Dept. of Biostatistics & Epidemiology , Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran. ,memohammadi@yahoo.com
3- M.Sc in Occupational Health Engineering, Research Assistant, Occupational Health Engineering, Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
4- M.Sc in Occupational Health and Safety Engineering, Dept. of Occupational Health and Safety Engineering, Student Research Committee, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Professor in Biostatistics, Dept. of Biostatistics & Epidemiology , Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran. ,
3- M.Sc in Occupational Health Engineering, Research Assistant, Occupational Health Engineering, Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
4- M.Sc in Occupational Health and Safety Engineering, Dept. of Occupational Health and Safety Engineering, Student Research Committee, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran.
Article history


Received: 2023/01/3
Accepted: 2023/05/30
ePublished: 2023/06/28
Accepted: 2023/05/30
ePublished: 2023/06/28
Subject:
Occupational Health
Keywords: N-Acetylcysteine [MeSH], Noise [MeSH], Temporary [MeSH], Permanent [MeSH], Hearing Loss [MeSH]
Full-Text [PDF 576 kb]
(445 Downloads)
| Abstract (HTML) (1548 Views)
.jpg)
Fig.1. A flowchart of the experimental protocol via DPOAE measurements, noise trauma and NAC injections
.jpg)
Fig. 2. Noise exposure chamber and noise generation system
.jpg)
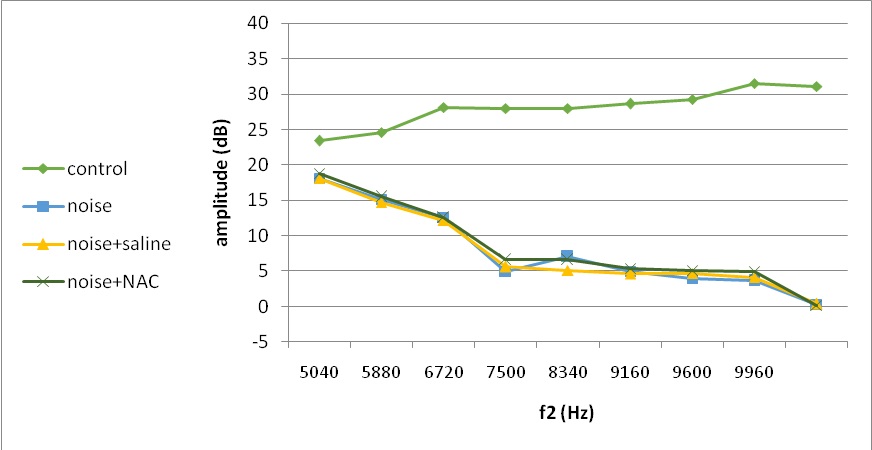
Fig. 4. Mean DPOAE amplitudes across 4620–9960 Hz measured at 1 days following noise exposure
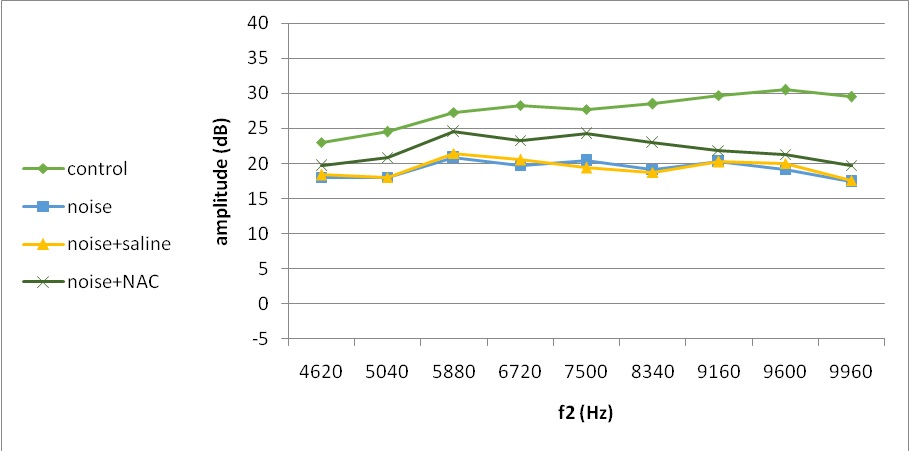
Fig. 5. Mean DPOAE amplitudes across 4620–9960 Hz measured at 7 days following noise exposure
Table 1. Mean (standard deviation) of DPOAE amplitude difference between post-exposure measured time points in the control and noise groups
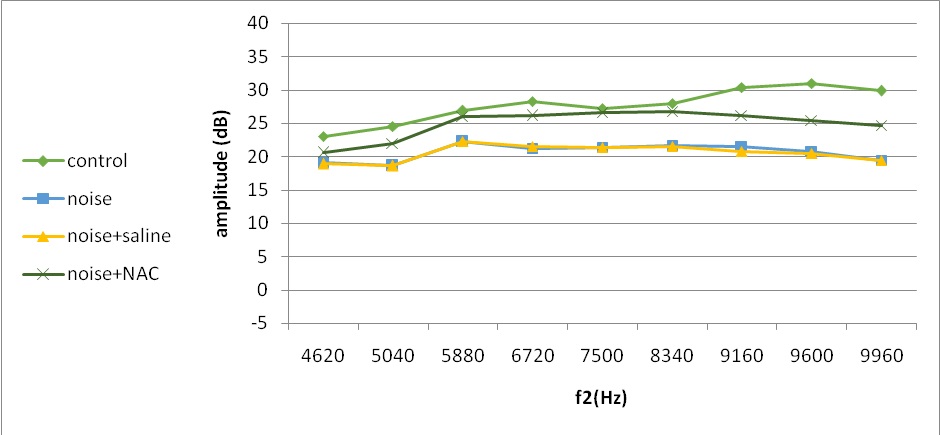
Fig. 6. Mean DPOAE amplitudes across 4620–9960 Hz measured at 21 days following noise exposure
Full-Text: (198 Views)
Introduction
One of the most prevalent forms of occupational disability globally is hearing loss. Leading causes of hearing impairment at work have been identified as exposure to noise and ototoxic substances [1]. Over 10% of people who work across the globe have hearing loss as a result of workplace noise[2].Based on WHO report in 2017, exposure to noise in recreational settings causes hearing loss in 1.1 billion young people (aged 12-35 years) [3].
One of the major mechanisms underlying noise-induced hearing loss (NIHL) is metabolic injury to the organ of corti (OC(. Exposure to moderate level of noise results in some biochemical changes in the hair cells leading eventually to death or damage to sensory epitilium. Noise causes overdriving of mitochondria, excitotoxicity and ischemia in the inner ear which all contribute to the generation of reactive oxygen species (ROS). The excessive levels of ROS causes hair cell lesion by inducing DNA and protein damage directly or through apoptetic cell death indirectly, and lipid peroxidation leading to necrotic cell death. This whole chemical process can explain hearing loss [4, 5]. There is also evidence that noise exposure elevates free radicals in industrial workers and also the cochlea of animals [6].
The formation of free radicals in the cochlea in terms of noise exposure suggested that vulnerability to NIHL can be influenced by endogenous antioxidant agents. N-Acetylcysteine (NAC) can directly scavenge free radicals, including ROS which has been safely applied to treat diseases, such as chronic bronchitis and keratoconjunctivitissicca. NAC also has the benefit of increasing intracellular glutathione (GSH) levels, which likewise function as free radical scavengers [7, 8]. When exposed to noise, ROS causes damage to the cochlea's hair cells. As a result, there is a brief period of transient hearing loss, which eventually turns into permanent hearing loss [9]. Although NAC has protective effect on hearing impairment, it cannot provide a complete recovery of baseline hearing levels measured prior to noise exposure [6]. The effect of NAC on noise-induced hearing loss has remained controversial. Some studies confirmed the permanent protective effect of NAC with no temporary influence on hearing [10-12]. While others reported immediate hearing threshold shift reduction by NAC after noise exposure [13-15]. These contentious findings could be attributed to differences in animal species, noise types, a wide range of noise exposure durations, exposure intensities, doses of NAC, timing and scheduling of injections, assessments of NIHL at various post-exposure time intervals, and the use of various assessment techniques [16–22]. On the other side in one study reported that antioxidant therapy is not successful to improve the established hearing loss [23].
Most of previous studies applied impulse noise (113-160 dB), acute exposure (30min-5hr) with octave band centered at 4 to 6 kHz, different animal species, and auditory brainstem response (ABR) hearing test [24, 25]. There are some studies on animals exposed to moderate intensity of noise (105 dB) just for 4-6 hr [16, 21]. However, workers are usually exposed to lower decibel of broad band noise over a long period of time in real workplaces.
The current study tested the hypotheses that NAC can protect the cochlea against high pass white noise centered at 8kHz trauma for 10 consecutive days in rat, and the dose schedule of NAC treatment determines the degree of protection of NAC against temporary and permanent hearing loss by distortion product otoacoustic emission (DPOAE) test before, 1, 7 and 21 days after noise exposure.
Materials and Methods
Animal models: In this experimental study, 24 adult male wistar rats (275±25 gr) were examined for no evidence of middle ear infection by otoscopic examination. Animals were obtained from Animal Research Center of Zahedan University of Medical Sciences (Iran). Animals were housed in polypropylene cages 40×20×15 cm for a week before experiments commence to be acclimatized. Except during exposure, food (rodent chow, Pars Animal Co, Iran), and tap water were given ad libitum. Lighting was on for 12 hours (07:00-19:00). Animal quarter temperature was 21-23oC and relative humidity ranged 40-50% [26]. Animals were daily transferred for the experiment and returned back to their home cages afterwards.
It was seriously taken to reduce animal suffering, as well as the number needed for the experiment. All procedures for the care and use of animals were approved by the Ethics Committee for Experimental Medicine of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1392.6662) based on following guidelines of the declaration of Helsinki.
Experimental protocol and NAC administration: A total of 24 rats were randomly assigned into 4 groups of 6 animals. The sample size was determined using 5% type I error, 5% type II error, and expecting a mean difference of 15 DPOAE by exposing rats to NAC in addition to noise [13] .
Figure 1 presents a flowchart of the experiment protocol. Control group (Group I) was neither exposed to noise nor to any dose of antioxidant. Noise group (Group II) was exposed to 102±0.5 dB [11, 21, 27] continuous high pass white noise centered at 8 kHz. Noise and NAC group (Group III) was exposed to noise, and received NAC (Zambon S.P.A, Italy) intraperitoneally, (i.p) 14 times (325 mg/kg/per injection); two days pre-noise once daily, 1 h pre-noise for 10 consecutive days, 2 days post-noise daily. Noise and Saline group (Group IV) was exposed to noise and received saline (vehicle of NAC) at a similar volume of NAC over the same schedule of noise and NAC group.
The best procedure described in the literature served as the foundation for the administration of NAC. This medication dosage is not ototoxic and does not result in long-term hearing loss when injected [11, 16]. Due to the possibility that the injection itself may induce hearing loss, Group IV acts as a control for Group III.
One of the most prevalent forms of occupational disability globally is hearing loss. Leading causes of hearing impairment at work have been identified as exposure to noise and ototoxic substances [1]. Over 10% of people who work across the globe have hearing loss as a result of workplace noise[2].Based on WHO report in 2017, exposure to noise in recreational settings causes hearing loss in 1.1 billion young people (aged 12-35 years) [3].
One of the major mechanisms underlying noise-induced hearing loss (NIHL) is metabolic injury to the organ of corti (OC(. Exposure to moderate level of noise results in some biochemical changes in the hair cells leading eventually to death or damage to sensory epitilium. Noise causes overdriving of mitochondria, excitotoxicity and ischemia in the inner ear which all contribute to the generation of reactive oxygen species (ROS). The excessive levels of ROS causes hair cell lesion by inducing DNA and protein damage directly or through apoptetic cell death indirectly, and lipid peroxidation leading to necrotic cell death. This whole chemical process can explain hearing loss [4, 5]. There is also evidence that noise exposure elevates free radicals in industrial workers and also the cochlea of animals [6].
The formation of free radicals in the cochlea in terms of noise exposure suggested that vulnerability to NIHL can be influenced by endogenous antioxidant agents. N-Acetylcysteine (NAC) can directly scavenge free radicals, including ROS which has been safely applied to treat diseases, such as chronic bronchitis and keratoconjunctivitissicca. NAC also has the benefit of increasing intracellular glutathione (GSH) levels, which likewise function as free radical scavengers [7, 8]. When exposed to noise, ROS causes damage to the cochlea's hair cells. As a result, there is a brief period of transient hearing loss, which eventually turns into permanent hearing loss [9]. Although NAC has protective effect on hearing impairment, it cannot provide a complete recovery of baseline hearing levels measured prior to noise exposure [6]. The effect of NAC on noise-induced hearing loss has remained controversial. Some studies confirmed the permanent protective effect of NAC with no temporary influence on hearing [10-12]. While others reported immediate hearing threshold shift reduction by NAC after noise exposure [13-15]. These contentious findings could be attributed to differences in animal species, noise types, a wide range of noise exposure durations, exposure intensities, doses of NAC, timing and scheduling of injections, assessments of NIHL at various post-exposure time intervals, and the use of various assessment techniques [16–22]. On the other side in one study reported that antioxidant therapy is not successful to improve the established hearing loss [23].
Most of previous studies applied impulse noise (113-160 dB), acute exposure (30min-5hr) with octave band centered at 4 to 6 kHz, different animal species, and auditory brainstem response (ABR) hearing test [24, 25]. There are some studies on animals exposed to moderate intensity of noise (105 dB) just for 4-6 hr [16, 21]. However, workers are usually exposed to lower decibel of broad band noise over a long period of time in real workplaces.
The current study tested the hypotheses that NAC can protect the cochlea against high pass white noise centered at 8kHz trauma for 10 consecutive days in rat, and the dose schedule of NAC treatment determines the degree of protection of NAC against temporary and permanent hearing loss by distortion product otoacoustic emission (DPOAE) test before, 1, 7 and 21 days after noise exposure.
Materials and Methods
Animal models: In this experimental study, 24 adult male wistar rats (275±25 gr) were examined for no evidence of middle ear infection by otoscopic examination. Animals were obtained from Animal Research Center of Zahedan University of Medical Sciences (Iran). Animals were housed in polypropylene cages 40×20×15 cm for a week before experiments commence to be acclimatized. Except during exposure, food (rodent chow, Pars Animal Co, Iran), and tap water were given ad libitum. Lighting was on for 12 hours (07:00-19:00). Animal quarter temperature was 21-23oC and relative humidity ranged 40-50% [26]. Animals were daily transferred for the experiment and returned back to their home cages afterwards.
It was seriously taken to reduce animal suffering, as well as the number needed for the experiment. All procedures for the care and use of animals were approved by the Ethics Committee for Experimental Medicine of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1392.6662) based on following guidelines of the declaration of Helsinki.
Experimental protocol and NAC administration: A total of 24 rats were randomly assigned into 4 groups of 6 animals. The sample size was determined using 5% type I error, 5% type II error, and expecting a mean difference of 15 DPOAE by exposing rats to NAC in addition to noise [13] .
Figure 1 presents a flowchart of the experiment protocol. Control group (Group I) was neither exposed to noise nor to any dose of antioxidant. Noise group (Group II) was exposed to 102±0.5 dB [11, 21, 27] continuous high pass white noise centered at 8 kHz. Noise and NAC group (Group III) was exposed to noise, and received NAC (Zambon S.P.A, Italy) intraperitoneally, (i.p) 14 times (325 mg/kg/per injection); two days pre-noise once daily, 1 h pre-noise for 10 consecutive days, 2 days post-noise daily. Noise and Saline group (Group IV) was exposed to noise and received saline (vehicle of NAC) at a similar volume of NAC over the same schedule of noise and NAC group.
The best procedure described in the literature served as the foundation for the administration of NAC. This medication dosage is not ototoxic and does not result in long-term hearing loss when injected [11, 16]. Due to the possibility that the injection itself may induce hearing loss, Group IV acts as a control for Group III.
.jpg)
Fig.1. A flowchart of the experimental protocol via DPOAE measurements, noise trauma and NAC injections
Test of hearing: Hearing was assessed via measuring distortion product otoacoustic emissions (DPOAEs) while animals were under general anesthesia by receiving a mixture of 68 mg/kg ketamine and 8mg/kg xylazine, intraperitoneally. In order to minimize the impact of standing waves in the external meatus, a frequency range of 4.0–10 kHz was taken into consideration for the bandwidth of the cubic DPOAE responses (2f1–f2, referred to f2). Twelve points were sampled each octave. The primary frequency tone ratio (f2/f1) was taken to be 1.21. A non-symmetrical DPOAE protocol was used to evoke the responses based on unequal primary tone stimulus intensities, i.e. L1 > L2. The hearing loss can be more precisely evaluated via such protocols [26, 28]
The protocols were defined as: high level (L1 = 60 and L2 = 50 dB SPL). All measurements equal or more than 3 dB for SNR were used for analysis. A heating blanket was used to keep the animal body temperature between 37.5-38.58oC. DPOAE was measured before intervention (baseline) and 1, 7, and 21 days after noise exposure. All measurements were performed inside a small sound-attenuated chamber lined with acoustic foam tiles.
Noise exposure: For noise exposure, each of 6 animals in a group was transferred, and put in a separate wire mesh cage 40×25×15 cm3and positioned on shelves inside a ventilated sound exposure custom-made reverberant chamber and were exposed to a high pass white noise centered at 8 kHz at 102 decibels sound pressure level (dB SPL) for 8 hours a day in 10 consecutive days.
The chamber dimensions was 60×45×30cm [29] (Fig. 2) and the primary design was based on proposed consideration in previous studies including; reasonability, practicality, good feasibility of test animal activity, ease of maintenance, controlling conditions for the temperature and humidity and maintaining a continuous flow of fresh air [30-33].
Noise was generated by Filtered Noise Generator software (TimoEsser's Audio software, version 1.2), and delivered by cool edit pro v. 2.1 made in Syntrillium Software Corporation. An amplifier (model: Rock Jw-s317, China) was used to intensify the generated noise. Then, noise propagation was performed by four loud speakers (type: Micro Lab, model: HT 25tweeter, Italy) mounted 0.15 meter above the animal cages at multiple locations inside the chamber to ensure stimulus uniformity by a maximum of 0.5 dB across. Four 1.2 cm diameter apertures were pierced and inserted on each side of the chamber, and a sound level meter (cel-450, type1, D, Casella-CEL) outfitted with an analyzer was used to measure the sound level inside the chamber.
DPOAEs were described as mean (SD). Normal distribution of data was confirmed by Shapiro-Wilk test. Two independent samples t-test, repeated measures ANOVA with simple method for doing contrasts were used for data analysis. The significance level was taken 0.05 and all data analysis was performed using SPSS (version 18).
The protocols were defined as: high level (L1 = 60 and L2 = 50 dB SPL). All measurements equal or more than 3 dB for SNR were used for analysis. A heating blanket was used to keep the animal body temperature between 37.5-38.58oC. DPOAE was measured before intervention (baseline) and 1, 7, and 21 days after noise exposure. All measurements were performed inside a small sound-attenuated chamber lined with acoustic foam tiles.
Noise exposure: For noise exposure, each of 6 animals in a group was transferred, and put in a separate wire mesh cage 40×25×15 cm3and positioned on shelves inside a ventilated sound exposure custom-made reverberant chamber and were exposed to a high pass white noise centered at 8 kHz at 102 decibels sound pressure level (dB SPL) for 8 hours a day in 10 consecutive days.
The chamber dimensions was 60×45×30cm [29] (Fig. 2) and the primary design was based on proposed consideration in previous studies including; reasonability, practicality, good feasibility of test animal activity, ease of maintenance, controlling conditions for the temperature and humidity and maintaining a continuous flow of fresh air [30-33].
Noise was generated by Filtered Noise Generator software (TimoEsser's Audio software, version 1.2), and delivered by cool edit pro v. 2.1 made in Syntrillium Software Corporation. An amplifier (model: Rock Jw-s317, China) was used to intensify the generated noise. Then, noise propagation was performed by four loud speakers (type: Micro Lab, model: HT 25tweeter, Italy) mounted 0.15 meter above the animal cages at multiple locations inside the chamber to ensure stimulus uniformity by a maximum of 0.5 dB across. Four 1.2 cm diameter apertures were pierced and inserted on each side of the chamber, and a sound level meter (cel-450, type1, D, Casella-CEL) outfitted with an analyzer was used to measure the sound level inside the chamber.
DPOAEs were described as mean (SD). Normal distribution of data was confirmed by Shapiro-Wilk test. Two independent samples t-test, repeated measures ANOVA with simple method for doing contrasts were used for data analysis. The significance level was taken 0.05 and all data analysis was performed using SPSS (version 18).
.jpg)
Fig. 2. Noise exposure chamber and noise generation system
Results
In all frequencies, four studied groups were not significantly different in terms of pre exposure baseline amplitudes (P>0.05) (Fig.3). In all experimental groups, DPOAE amplitudes measured at either 1, 7, or 21 days after exposure were significantly different with the baseline among all frequencies (P<0.05).
In all frequencies, four studied groups were not significantly different in terms of pre exposure baseline amplitudes (P>0.05) (Fig.3). In all experimental groups, DPOAE amplitudes measured at either 1, 7, or 21 days after exposure were significantly different with the baseline among all frequencies (P<0.05).
.jpg)
Fig. 3. Mean DPOAE amplitudes across 4620–9960 Hz measured at pre-noise exposure
One day after the intervention, DPOAE amplitude decreased in all experimental groups (noise, noise and saline, and noise and NAC) relative to the control group (Group I) by increasing frequency by about 5 to 30 dB (Fig. 4). This temporary change was significantly different between noise exposed group with the control group, as well as the noise and saline group with noise and NAC group.

Fig. 4. Mean DPOAE amplitudes across 4620–9960 Hz measured at 1 days following noise exposure
At 7 days post-exposure, amplitudes were improved in all experimental groups, especially in noise and NAC group with more recovery in low and middle frequencies (Fig. 5). In noise-exposed animals, the first recovery roughly ranged from 0 to 17 by frequency increase, which was substantially different between the noise group and controls (Table 1). However, there was no discernible difference between the noise and saline or noise and NAC groups of mice (Table 2).

Fig. 5. Mean DPOAE amplitudes across 4620–9960 Hz measured at 7 days following noise exposure
Table 1. Mean (standard deviation) of DPOAE amplitude difference between post-exposure measured time points in the control and noise groups
| Frequency (Hz) |
7th day – 1st day (initial recovery) |
21st -7th day (subsequent recovery) |
1st -1th day (total recovery) |
||||||
| Control | Noise | P_value* | Control | Noise | P_value* | Control | Noise | P_value* | |
| 4620 | -0.38 (2.21) |
-0.08 (1.28) |
0.822 | 0.00 (1.15) |
1.75 (0.88) |
0.134 | -0.38 (1.11) |
1.08 (1.20) |
0.089 |
| 5040 | -0.12 (1.03) |
2.88 (1.31) |
0.011 | 0.00 (1.15) |
0.62 (0.25) |
0.362 | -0.12 (0.25) |
3.50 (1.15) |
0.007 |
| 5880 | -0.88 (0.75) |
8.20 (2.17) |
<0.001 | -0.38 (2.14) |
1.40 (2.19) |
0.262 | -1.25 (2.78) |
9.60 (3.78) |
0.002 |
| 6720 | 0.25 (1.50) |
14.75 (2.22) |
<0.001 | 0.00 (1.82) |
1.50 (0.41) |
0.199 | 0.25 (1.26) |
16.25 (2.33) |
<0.001 |
| 7500 | -0.25 (1.85) |
14.00 (3.32) |
<0.001 | -0.50 (1.68) |
1.60 (1.52) |
0.090 | -0.75 (2.06) |
15.60 (2.61) |
<0.001 |
| 8340 | -0.12 (2.29) |
14.20 (4.31) |
0.001 | -0.50 (1.91) |
2.5 (2.55) |
0.093 | -0.62 (2.25) |
16.70 (3.15) |
<0.001 |
| 9180 | 0.50 (1.91) |
16.25 (2.84) |
<0.001 | 0.62 (2.84) |
1.25 (0.64) |
0.694 | 1.12 (3.35) |
17.50 (2.89) |
<0.001 |
| 9600 | -1.00 (1.41) |
15.60 (3.27) |
<0.001 | 0.50 (0.58) |
1.60 (1.34) |
0.174 | -0.50 (1.73) |
17.20 (2.36) |
<0.001 |
| 9960 | -1.62 (3.57) |
17.10 (2.36) |
<0.001 | 0.38 (0.75) |
2.00 (2.24) |
0.211 | -1.25 (3.10) |
19.10 (0.55) |
<0.001 |
Significant effects (P < 0.05) are marked in bold
Table 2. Mean (standard deviation) of DPOAE amplitude difference between post-exposure measured time points in the noise +saline and noise+NAC groups
Table 2. Mean (standard deviation) of DPOAE amplitude difference between post-exposure measured time points in the noise +saline and noise+NAC groups
| Frequency (Hz) |
7th day – 1st day (initial recovery) |
21st -7th day (subsequent recovery) |
1st -1th day (total recovery) |
||||||
| Noise+ saline | Noise+ NAC | P_value* | Noise+ saline | Noise+ NAC | P_value* | Noise+ saline | Noise+ NAC | P_value* | |
| 4620 | 0.30 (1.20) |
0.83 (2.84) |
0.706 | 0.50 (2.12) |
0.92 (2.27) |
0.762 | 0.80 (2.95) | 1.75 (1.54) |
0.508 |
| 5040 | 3.25 (2.53) |
5.40 (2.77) |
0.269 | 0.62 (0.48) |
1.10 (0.55) |
0.209 | 3.88 (2.62) | 6.50 (2.78) |
0.193 |
| 5880 | 9.20 (2.39) |
12.00 (4.60) |
0.253 | 0.90 (0.22) |
1.50 (1.05) |
0.225 | 10.10 (2.46) | 13.50 (4.14) | 0.142 |
| 6720 | 14.88 (2.59) |
16.75 (2.86) |
0.324 | 1.00 (0.82) |
2.75 (3.13) |
0.314 | 15.88 (2.02) | 19.50 (2.49) | 0.042 |
| 7500 | 14.30 (1.86) |
17.67 (2.93) |
0.054 | 2.00 (1.00) |
2.42 (1.02) |
0.513 | 16.30 (1.10) | 20.08 (3.11) | 0.030 |
| 8340 | 14.10 (4.26) |
17.50 (4.34) |
0.224 | 2.85 (1.73) |
3.83 (4.24) |
0.641 | 16.95 (1.64) | 21.33 (1.97) | 0.003 |
| 9180 | 15.50 (3.08) |
16.75 (1.84) |
0.425 | 0.60 (1.52) |
4.33 (1.03) |
0.001 | 16.10 (3.32) | 21.08 (1.80) | 0.011 |
| 9600 | 15.90 (1.47) |
16.33 (1.86) |
0.684 | 0.50 (2.00) |
4.08 (1.80) |
0.012 | 16.40 (1.85) | 20.42 (0.49) | 0.001 |
| 9960 | 17.20 (2.56) |
19.58 (3.73) |
0.259 | 1.80 (1.79) |
4.92 (0.74) |
0.004 | 19.00 (1.17) | 24.50 (3.54) | 0.009 |
Significant effects (P < 0.05) are marked in bold
Subsequent recovery between 7 and 21 days post-exposure was even promoted in all experimental groups (Fig. 6). However, more than three times degree of [improvement (P<0.05) occurred in higher frequencies for animals treated with NAC compared to those received either noise only or noise and saline (Table 2).

Fig. 6. Mean DPOAE amplitudes across 4620–9960 Hz measured at 21 days following noise exposure
The most part of therapeutic recovery occurred between 1 and 7 days of experiment. The total recovery over 21 days after acoustic trauma approximately ranged 1-19 by frequency increase in the noise, and noise and saline groups compared to 1.75-24.50 in animals receiving NAC (Table 2). Even though all experimental groups saw about the same rate of recovery at lower frequencies (4620–5880 Hz), animals given NAC had considerably greater amplitudes at higher frequencies (6720–9960 Hz). Despite total recovery over 21 days after noise exposure, amplitudes were still significantly different with the baseline even in noise exposed animals treated with NAC (P<0.05) (Fig. 6). Noise and NAC group showed less permanent hearing loss (P<0.05) than animals exposed only to noise or noise and saline group (2-5 vs. 4-12 dB).
Discussion
The current study’s results showed a significant reduction in DPOAE amplitude after exposing to noise regardless of receiving antioxidant. However, the NAC treated animals experienced more improvement in the recovery of DPOAE level between one and 21 days after noise exposure with more changes in higher frequencies (6720-9960 Hz). The most elevation of amplitude occurred at 7 days post-exposure followed by steady improvement until 21 days.
There is evidence that noise exposure causes delayed free radical formation which peaks within 12 hours of post-exposure. This leads to NIHL and sensory cell death which can be mostly prevented by pre-treatment [21]. NAC was administered as a pre-treatment in the present research twice daily, one hour before to noise exposure, and once daily for two days after noise exposure. Following exposure to noise, treated and untreated rats with NAC both had temporary hearing loss (within 1 day of exposure). However, protective effect of NAC was significant on permanent hearing loss over 21 days post-exposure. These results are in agreement with previous studies findings that indicated the effect of NAC on permanent threshold shift (PTS) reduction, but no effect on temporary threshold shift (TTS) in noise-exposed guinea [10, 34] chinchillas [11] and man rodents [4]. Yamasobaet al. [35] reported that oxothiazolidine-4-carboxylate (OTC), a procysteine drug which promotes rapid restoration of GSH level, could reduce PTS but TTS remained unchanged after noise exposure (102-dB SPL, 3 h a day for 5 consecutive days). In another study, sodium salicylate (SAL), and NAC were not able to improve TTS in chinchilla (105 dB centered at 4kHz, 6hr exposure) [16]. In contrast, some studies illustrated that NAC may prevent or reduce temporary and permanent hearing loss in rabbits and humans [13, 14]. Moreover, Davis et al. [36] reported that L-NAC was not effective in preventing permanent hearing loss in mouse.
Despite differences in the degree and length of noise exposure, medication dosage schedules, and medicines that increase endogenous GSH level only provide little protection. This may be supported by data showing that hydroxyl scavengers partially reduce lipid peroxidation by competing for OH in 'free solution' rather than penetrating lipid membrane barriers to scavenge 0OH within cells [4]. There is evidence that hydroxyl radicals can be suppressed by some lipid soluble antioxidants, such as Coenzyme Q10 (CoQ10) through inhibiting production of 4-Hydroxy-2-nonenal (4-HNE) which is the metabolic of the hydroxyl radical [37]. The prevention of cochlear damage may be more effective by multiple antioxidants targeting different pathways of noise-induced ototoxicity because different antioxidants work through different mechanisms [38].
In noised only exposed animals, DPOAE amplitudes were partially improved within 1 to 21 days after exposure. This results which are consistent with the findings in many studies with similar designs reflect some reversible structural changes due to noise exposure [39-43]. The causes of reversible hearing loss have been demonstrated in animal studies on acoustic damage, including swelling of afferent nerve fibers and endings beneath the bases of inner hair cells (IHCs), bent or collapsed pillars, pillar buckling that results in the separation of the outer hair cell's (OHC) sterocilium from the tectorial membrane (TM) and a reduction in hair cell stimulation, and partially collapsed supporting cells that cause the OC' Therefore, the difference between baseline DPOAEs, and those measured at 21 days after exposure may reflect permanent changes, such as collapse, fusion, fracturing of roots and complete loss in IHCs and OHCs stereocilia [44, 45].
Based on the cochlear physiology, a certain area of cochlea is stimulated by specific frequency in a sound wave. By increasing the frequency of sound stimulation, a closer part of the basal region of the cochlea is stimulated [46]. In this study, instrumental limitation of frequencies below 10 kHz did not allow for further assessment in the higher frequency basal region of the cochlea. Therefore, greater undetectable hearing loss in this region of the cochlea are possible in terms of upper limit of the region of auditory trauma [6, 47].
In the noise exposed animals, the most of DPOAE level changes was measured in higher frequencies (6720-9960 Hz) by increasing more susceptibility, and hair cells disturbing of the cochlea region responsible for transuding of higher sound frequencies. The more vulnerability can be due to several reasons: (1) apex-to-base gradient in swelling of baseline dendritic in the IHC area [47], (2) smaller volume of scala tympani in lower basal turn compared to middle and apex due to proximity to round window results in perilymph space reduction, and disability to quick dilution of ROS released by sensory epithelium [47], (3) GSH, as an antioxidant, is lower in basal OHCs compared to apical OHCs which could be elevated by administrating of NAC [48]. The latter could be explained by several known mechanisms including scavenging free radicals, production of GSH, protection of mitochondria, inhibition of glutamate excitotoxicity, lipid peroxidation and necrosis [17, 49].
DPOAE levels at the end of the research compared to baseline values show that NAC partly restored cochlear function, which resulted in partial preservation of OHCs. Another research on guinea pigs revealed similar outcomes [6].Our study had some limitations and therefore, our findings should be interpreted carefully. This study could not investigate hearing changes at frequencies higher than 10,000 Hz in terms of the instrumental limitations. As more hearing attenuation was detected at higher frequencies after 1 day of exposure, [46, 47] use of instruments with a greater broad frequency range is recommended to obtain more accurate results about the effects of NAC against nihl. Incapability assessment ofthreshold levels by the Ecolab labat instrument was another limitation in this study.
Conclusion
NAC as a water soluble antioxidant was able to partially improve permanent hearing loss after noise exposure, however, temporary hearing loss was not affected by NAC. By focusing on several routes in the generation of ROS and RNS, NAC in combination with other antioxidants such the lipid soluble antioxidant group, CoQ10, vitamin E, and idebenone may provide greater protection against hearing loss.
Acknowledgement
Authors would like to thank Zahedan University of Medical Sciences (Zahedan, Iran) for scientific and financial support.
Conflict of interest: None declared.
Discussion
The current study’s results showed a significant reduction in DPOAE amplitude after exposing to noise regardless of receiving antioxidant. However, the NAC treated animals experienced more improvement in the recovery of DPOAE level between one and 21 days after noise exposure with more changes in higher frequencies (6720-9960 Hz). The most elevation of amplitude occurred at 7 days post-exposure followed by steady improvement until 21 days.
There is evidence that noise exposure causes delayed free radical formation which peaks within 12 hours of post-exposure. This leads to NIHL and sensory cell death which can be mostly prevented by pre-treatment [21]. NAC was administered as a pre-treatment in the present research twice daily, one hour before to noise exposure, and once daily for two days after noise exposure. Following exposure to noise, treated and untreated rats with NAC both had temporary hearing loss (within 1 day of exposure). However, protective effect of NAC was significant on permanent hearing loss over 21 days post-exposure. These results are in agreement with previous studies findings that indicated the effect of NAC on permanent threshold shift (PTS) reduction, but no effect on temporary threshold shift (TTS) in noise-exposed guinea [10, 34] chinchillas [11] and man rodents [4]. Yamasobaet al. [35] reported that oxothiazolidine-4-carboxylate (OTC), a procysteine drug which promotes rapid restoration of GSH level, could reduce PTS but TTS remained unchanged after noise exposure (102-dB SPL, 3 h a day for 5 consecutive days). In another study, sodium salicylate (SAL), and NAC were not able to improve TTS in chinchilla (105 dB centered at 4kHz, 6hr exposure) [16]. In contrast, some studies illustrated that NAC may prevent or reduce temporary and permanent hearing loss in rabbits and humans [13, 14]. Moreover, Davis et al. [36] reported that L-NAC was not effective in preventing permanent hearing loss in mouse.
Despite differences in the degree and length of noise exposure, medication dosage schedules, and medicines that increase endogenous GSH level only provide little protection. This may be supported by data showing that hydroxyl scavengers partially reduce lipid peroxidation by competing for OH in 'free solution' rather than penetrating lipid membrane barriers to scavenge 0OH within cells [4]. There is evidence that hydroxyl radicals can be suppressed by some lipid soluble antioxidants, such as Coenzyme Q10 (CoQ10) through inhibiting production of 4-Hydroxy-2-nonenal (4-HNE) which is the metabolic of the hydroxyl radical [37]. The prevention of cochlear damage may be more effective by multiple antioxidants targeting different pathways of noise-induced ototoxicity because different antioxidants work through different mechanisms [38].
In noised only exposed animals, DPOAE amplitudes were partially improved within 1 to 21 days after exposure. This results which are consistent with the findings in many studies with similar designs reflect some reversible structural changes due to noise exposure [39-43]. The causes of reversible hearing loss have been demonstrated in animal studies on acoustic damage, including swelling of afferent nerve fibers and endings beneath the bases of inner hair cells (IHCs), bent or collapsed pillars, pillar buckling that results in the separation of the outer hair cell's (OHC) sterocilium from the tectorial membrane (TM) and a reduction in hair cell stimulation, and partially collapsed supporting cells that cause the OC' Therefore, the difference between baseline DPOAEs, and those measured at 21 days after exposure may reflect permanent changes, such as collapse, fusion, fracturing of roots and complete loss in IHCs and OHCs stereocilia [44, 45].
Based on the cochlear physiology, a certain area of cochlea is stimulated by specific frequency in a sound wave. By increasing the frequency of sound stimulation, a closer part of the basal region of the cochlea is stimulated [46]. In this study, instrumental limitation of frequencies below 10 kHz did not allow for further assessment in the higher frequency basal region of the cochlea. Therefore, greater undetectable hearing loss in this region of the cochlea are possible in terms of upper limit of the region of auditory trauma [6, 47].
In the noise exposed animals, the most of DPOAE level changes was measured in higher frequencies (6720-9960 Hz) by increasing more susceptibility, and hair cells disturbing of the cochlea region responsible for transuding of higher sound frequencies. The more vulnerability can be due to several reasons: (1) apex-to-base gradient in swelling of baseline dendritic in the IHC area [47], (2) smaller volume of scala tympani in lower basal turn compared to middle and apex due to proximity to round window results in perilymph space reduction, and disability to quick dilution of ROS released by sensory epithelium [47], (3) GSH, as an antioxidant, is lower in basal OHCs compared to apical OHCs which could be elevated by administrating of NAC [48]. The latter could be explained by several known mechanisms including scavenging free radicals, production of GSH, protection of mitochondria, inhibition of glutamate excitotoxicity, lipid peroxidation and necrosis [17, 49].
DPOAE levels at the end of the research compared to baseline values show that NAC partly restored cochlear function, which resulted in partial preservation of OHCs. Another research on guinea pigs revealed similar outcomes [6].Our study had some limitations and therefore, our findings should be interpreted carefully. This study could not investigate hearing changes at frequencies higher than 10,000 Hz in terms of the instrumental limitations. As more hearing attenuation was detected at higher frequencies after 1 day of exposure, [46, 47] use of instruments with a greater broad frequency range is recommended to obtain more accurate results about the effects of NAC against nihl. Incapability assessment ofthreshold levels by the Ecolab labat instrument was another limitation in this study.
Conclusion
NAC as a water soluble antioxidant was able to partially improve permanent hearing loss after noise exposure, however, temporary hearing loss was not affected by NAC. By focusing on several routes in the generation of ROS and RNS, NAC in combination with other antioxidants such the lipid soluble antioxidant group, CoQ10, vitamin E, and idebenone may provide greater protection against hearing loss.
Acknowledgement
Authors would like to thank Zahedan University of Medical Sciences (Zahedan, Iran) for scientific and financial support.
Conflict of interest: None declared.
References
1. Zhou JN, Shi ZH, Zhou LF, Hu Y, Zhang MB. Occupational noise-induced hearing loss in China: a systematic review and meta-analysis. BMJ Open. 2020;10(9):e039576. [DOI] [PMID] [PMCID]
2. Etemadinezhad S, Sammak Amani A, Moosazadeh M, Rahimlou M, Samaei SE. Occupational Noise-Induced Hearing Loss in Iran: A Systematic Review and Meta-Analysis. Iran J Public Health. 2023;52(2):278-89. [DOI] [PMID] [PMCID]
3. Sheffield AM, Smith RJH. The Epidemiology of Deafness. Cold Spring Harb Perspect Med. 2019;9(9):a033258. [DOI] [PMID] [PMCID]
4. Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226(1-2):22-43. [DOI] [PMID] [PMCID]
5. Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs. 2017;26(1):85-96. [DOI] [PMID] [PMCID]
6. Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29(2):70-5. [PMID] [PMCID]
7. Duan ML, Ulfendahl M, Laurell G, Counter SA, Pyykkö I, Borg E, et al. Protection and treatment of sensorineural hearing disorders caused by exogenous factors: experimental findings and potential clinical application. Hear Res. 2002;169(1-2):169-78. [DOI] [PMID]
8. Chang PH, Liu CW, Hung SH, Kang YN. Effect of N-acetyl-cysteine in prevention of noise-induced hearing loss: a systematic review and meta-analysis of randomized controlled trials. Arch Med Sci. 2021;18(6):1535-41 [DOI] [PMID] [PMCID]
9. 9. Müller J, Janssen T. Impact of occupational noise on pure-tone threshold and distortion product otoacoustic emissions after one workday. Hear Res. 2008;246(1-2):9-22. [DOI] [PMID]
10. Duan M, Qiu J, Laurell G, Olofsson A, Counter SA, Borg E. Dose and time-dependent protection of the antioxidant N-L-acetylcysteine against impulse noise trauma. Hear Res. 2004;192(1-2):1-9. [DOI] [PMID]
11. Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149(1-2):138-46. [DOI] [PMID]
12. Bai X, Wang M, Niu X, Yu H, Yue JX, Sun Y. Effect of N-acetyl-cysteine treatment on sensorineural hearing loss: a meta-analysis. World J Otorhinolaryngol Head Neck Surg. 2022;8(3):205-12. [DOI] [PMID] [PMCID]
13. Motalebi Kashani M, Saberi H, Hannani M. Prevention of Acoustic Trauma-Induced Hearing Loss by N-acetylcysteine Administration in Rabbits. Arch Trauma Res. 2013;1(4):145-50. [DOI] [PMID] [PMCID]
14. Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, Ma MC, et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269(1-2):42-7. [DOI] [PMID]
15. Rosenhall U, Skoog B, Muhr P. Treatment of military acoustic accidents with N-Acetyl-L-cysteine (NAC). Int J Audiol. 2019;58(3):151-7. [DOI] [PMID]
16. Coleman J, Huang X, Liu J, Kopke R, Jackson R. Dosing study on the effectiveness of salicylate/N-acetylcysteine for prevention of noise-induced hearing loss. Noise Health. 2010;12(48):159-65. [DOI] [PMID]
17. Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med. 1998;92(4):609-23. [DOI] [PMID]
18. Kashani MM, Khavanin A, Alameh A, Mirzaee R, Akbari M. Effects of N-acetylcysteine on auditory brainstem response threshold shift in rabbits exposed to noise and carbon monoxide. AJAS. 2010;7(6):201-7. [DOI]
19. Kopke R, Bielefeld E, Liu J, Zheng J, Jackson R, Henderson D, et al. Prevention of impulse noise-induced hearing loss with antioxidants. Acta Otolaryngol. 2005;125(3):235-43. [DOI] [PMID]
20. Kopke RD, Jackson RL, Coleman JKM, Liu J, Bielefeld EC, Balough BJ. NAC for noise: from the bench top to the clinic. Hearing Res. 2007;226(1):114-25. [DOI] [PMID]
21. Lorito G, Giordano P, Prosser S, Martini A, Hatzopoulos S. Noise-induced hearing loss: a study on the pharmacological protection in the Sprague Dawley rat with N-acetyl-cysteine. Acta Otorhinolaryngol Ital. 2006;26(3):133-9. [DOI] [PubMed]
22. Wu HP, Hsu CJ, Cheng TJ, Guo YL. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear Res. 2010;267(1-2):71-7. [DOI] [PMID]
23. Gill G, Blakley BW. Does N-acetylcysteine Improve Established Hearing Loss in Guinea Pigs? OTO Open. 2022;6(2):2473974X221100545. [DOI] [PMID] [PMCID]
24. Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966(2):265-73. [DOI] [PMID]
25. Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26. [DOI] [PMID] []
26. Pouyatos B, Campo P, Lataye R. Use of DPOAEs for assessing hearing loss caused by styrene in the rat. Hear Res. 2002;165(1-2):156-64. [DOI] [PMID]
27. Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, Bergamini C, et al. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33(9):4011-23. [DOI] [PMID] []
28. Hatzopoulos S, Petruccelli J, Laurell G, Finesso M, Martini A. Evaluation of anesthesia effects in a rat animal model using otoacoustic emission protocols. Hear Res. 2002;170(1-2):12-21. [DOI] [PMID]
29. Cobo P, Murillo-Cuesta S, Cediel R, Moreno A, Lorenzo-García P, Varela-Nieto I. Design of a reverberant chamber for noise exposure experiments with small animals. Appl acoustics. 2009;70(8):1034-40. [DOI]
30. Keith Prusaczyk W, Fischer G. A small-animalinhalation chamber for short-to-intermediate term exposure. Behav Res Methods Instrum. 1983;15(3):369-73. [DOI]
31. Laskin S, Drew RT. An inexpensive portable inhalation chamber. Am Ind Hyg Assoc J. 1970;31(5):645-6. [DOI] [PMID]
32. O'Shaughnessy PT, Achutan C, O'Neill ME, Thorne PS. A small whole-body exposure chamber for laboratory use. Inhal Toxicol. 2003;15(3):251-63. [DOI] [PMID]
33. Wong BA. Inhalation exposure systems: design, methods and operation. Toxicol Pathol. 2007;35(1):3-14. [DOI] [PMID]
34. Gonzalez-Gonzalez S, Cazevieille C. N-acetylcysteine Treatment Reduces Noise-induced Hearing Loss in Guinea Pig. J Community Prev Med. 2020;3(1):1-6. [DOI]
35. Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784(1-2):82-90. [DOI] [PubMed]
36. Davis RR, Custer DA, Krieg E, Alagramam K. N-Acetyl L-Cysteine does not protect mouse ears from the effects of noise. J Occup Med Toxicol. 2010;5:11. [DOI] [PMID] [Google Scholar]
37. Sugahara K, Hirose Y, Mikuriya T, Hashimoto M, Kanagawa E, Hara H, et al. Coenzyme Q10 protects hair cells against aminoglycoside. PLoS One. 2014;9(9):e108280 [DOI] [PMID] [PMCID]
38. Hullfish H, Roldan LP, Hoffer ME. The Use of Antioxidants in the Prevention and Treatment of Noise-Induced Hearing Loss. Otolaryngol Clin North Am. 2022;55(5):983-91. [DOI] [PMID]
39. Ahn JH, Joo HS, Suh JK, Kim H, So HS, Chung JW. Effects of cigarette smoking on hearing recovery from noise-induced temporary hearing threshold shifts in mice. Otol Neurotol. 2011;32(6):926-32. [DOI] [PMID]
40. Fetoni AR, Ferraresi A, Greca CL, Rizzo D, Sergi B, Tringali G, et al. Antioxidant protection against acoustic trauma by coadministration of idebenone and vitamin E. Neuroreport. 2008;19(3):277-81. [DOI] [PMID]
41. Fetoni AR, Piacentini R, Fiorita A, Paludetti G, Troiani D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL). Brain Res. 2009;1257:108-16. [DOI] [PMID]
42. Hougaard KS, Barrenäs ML, Kristiansen GB, Lund SP. No evidence for enhanced noise induced hearing loss after prenatal stress or dexamethasone. Neurotoxicol Teratol. 2007;29(6):613-21. [] [PMID]
43. Lu J, Li W, Du X, Ewert DL, West MB, Stewart C, et al. Antioxidants reduce cellular and functional changes induced by intense noise in the inner ear and cochlear nucleus. J Assoc Res Otolaryngol. 2014;15(3):353-72. [DOI] [PMID] [Google Scholar]
44. Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139(1-2):13-30. [DOI] [PMID]
45. Ada S, Hanci D, Ulusoy S, Vejselova D, Burukoglu D, Muluk NB, et al. Potential protective effect of N-acetyl cysteine in acoustic trauma: An experimental study using scanning electron microscopy. Adv Clin Exp Med. 2017;26(6):893-7. [DOI] [PMID]
46. Habybabady RH, Mohammadi M, Mortazavi SB, Khavanin A, Mirzaei R, Malvajerdi MS. The effect of simultaneous exposure to cigarette smoke and noise on distortion product otoacoustic emissions in rats. Toxicol Ind Health. 2019;35(5):349-57. [DOI] [PMID]
47. Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3(3):248-68. [DOI] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution 4.0 International License. |







