BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://johe.rums.ac.ir/article-1-421-en.html
Google Scholar
2- MPH, MSc, FWACP, FMCPH in Public Health, Consultant and Senior lecturer, Dept. of Community Medicine, University of Nigeria Teaching Hospital, Enugu, University of Nigeria, Nsukka, Nigeria.
3- MPH, MSc, FWACP in Public Health, Consultant Public Health Physician, Dept. of Community Medicine, Alex Ekwueme Federal University Teaching Hospital Abakaliki, Abakaliki, Nigeria.
4- Professor of Public Health, Dept. of Community Medicine, University of Nigeria Teaching Hospital, Enugu, Nigeria; University of Nigeria, Nsukka, Nigeria.

Accepted: 2021/03/9
ePublished: 2021/06/21
Introduction
Lead (Pb) is a ubiquitous natural-occurring heavy metal found in many occupational processes or activities in inorganic and organic forms [1, 2]. Human exposure occurs through activities, including fossil fuel burning, mining, and manufacturing [3]. Though the use of Pb is as old as humankind, its poisoning reached an epidemic dimension by the 19th and 20th centuries, influenced by industrialization and its uncontrolled use in pure or alloyed forms [4, 5]. Lead poisoning was known to be toxic in high doses, and the burden of toxicity was greatly underestimated since mostly the poisoning was clinically overt [3]. However, from the 20th century to the present, most developed countries have halted Pb poisoning by restricting it in industry and using unleaded gasoline, except chronic exposures at lower levels. The reverse is the case in developing countries, where there are non-existent biomonitoring programs [6, 7]. Lead enters the body through inhalation of dust and fumes, ingestion, and absorption by the skin [8 –10]. Pb poisoning effects are of respective target organs, e.g., haematologic, neurobehavioral, cardiotoxic, nephrotoxic, reproductive, and gastrointestinal effects [1, 11]. They occur in severe clinical and subclinical/asymptomatic forms [4].
Panel beaters are part of automobile technicians whose occupational activities of vehicle body repairs, cutting, soldering, and spray painting expose them to lead poisoning [12]. This occupational group operates in different forms and organizations. Some are observed along the roadsides with no safety rules, while others work in a formal sector governed by safety rules. [13] This setup may result in different occupational Pb exposure levels and haematoxicity effects.
The haematological effects of Pb poisoning occur following acute or chronic lead exposure interfering in the enzymatic steps in haeme synthesis pathway with a consequent reduction in red blood cells [1, 7, 14]. The impairment of haeme biosynthesis is first by inhibition of delta–aminolaevulinic acid dehydratase (ALA–D) with an elevation of protoporphyrin in the erythrocytes. This is dose-related, noted initially at a blood lead concentration of 10–20µg|dl and completed at levels of 70–90µg|dl. It is second by inhibition of ferrochelatase, causing increased excretion of coproporphyrin and aminolevulinic acid (ALA) in the urine. This begins to rise at a blood lead concentration of 25–30µg|dl [1, 15], confirmed by marked reductions in blood haemoglobin and haematocrit levels and other erythrocytes after Pb exposure [16]. Other haematologic effects are direct toxic action of lead on leucopoiesis in lymphoid organs with an increase in total leucocytes, basophils, eosinophils, and lymphocytes, as well as lead inhibition of platelets aggregation in the bone marrow with an increase in platelet and platelet distribution width [15, 17].
The commonest outcome of lead haematopoietic toxicity is anaemia. At acute high-level exposure, lead poisoning causes haemolytic anaemia, while at chronic low-level exposure, it causes frank anaemia [11]. According to the Agency for Toxic Substances and Disease Registry (ATSDR-1999), children and adults with lead levels of ≥70µg|dl and ≥ 80µg|dl, respectively, are anaemic. Also, Schwartz et al. (1990) noted that 20% of children with blood lead above 60µg|dl were anaemic [18]. A study in Iran found that the prevalence of anaemia among children at blood lead level <5µg|dl was 40%, while another study in Egypt found 63.3% of children with blood lead ≥10µg|dl [19, 20].
Few studies have been performed in Nigeria and other low-income countries on occupational Pb-induced haematoxicity, none of which has compared the haemtoxicity effects of Pb exposure between occupational groups with different organizational structures affecting exposure. Available studies have assessed the occupational Pb exposure at higher thresholds [5]. The present study helps occupational health physicians understand the relationship between lead exposure and haematological parameters (HP) at varying levels of exposure for early intervention. Further, it can add to existing local knowledge and data. It can also help ascertain differences, if any, among workers with different organizational structures and occupational health practices. Therefore, this study aims at assessing the relationship between haematological parameters and lead exposure among roadside and organized panel beaters in Enugu Metropolis, Nigeria.
Materials and Methods
The study area is the Enugu metropolis, the capital of Enugu State in the southeast geo-political zone of Nigeria. The metropolis is constituted by three Local Government Areas (LGA) in Enugu State, including Enugu North, Enugu South, and Enugu East, and inhabited mostly by the Igbo ethnic group [21]. The panel beaters in this part of the state do not have a centralized garage, i.e., mechanic village. The roadside (informal) panel beaters are observed mainly in the streets; some are on streets close to spare parts markets. The organized (formal) panel beaters are those under government rules governed by factory, labor law, and workman compensation acts. They have an organizational structure that promotes safety precautions.
This was a comparative cross-sectional study of roadside and organized panel beaters in the Enugu metropolis. In this research, panel beaters and trainees who were 18 years and above, spent over one year with an exposure time of 8 hours and above, not involved in another job, and interested in participating in the study were included, while those with chronic diseases were excluded.
The minimum sample size for the study was determined using the formula for comparing two independent proportions [22], as well as 14.3% representing the proportion of Nigerian adults with blood lead levels >20ug|dl [23]. Standard normal deviate (Zα) of 1.96% and 80% power (Zβ) were included. A minimum sample size of 214 per sector was obtained after correcting the non-response rate, giving a sample size of 428.
A multistage sampling technique was used for both roadside and organized panel beaters in this study. For the roadside panel beaters, the first stage was the selection of Enugu North among the three local government areas by simple random sampling as a zone using the balloting method. The second was the selection of one division out of the 5 in Enugu North LGA by simple random sampling using the balloting method. The third was the selection of 10 branches out of the 13 in Enugu North LGA by simple random sampling using the balloting method. Lastly, stratification and proportionate allocation of panel beaters from all the workshops within the selected branches were performed, and a total of 228 panel beaters were selected.
For the organized panel beaters, the first phase was the selection of Enugu North among the three local government areas by simple random sampling as a zone using the balloting method. The second was the proportionate allocation of government and private-owned company workers. There were a total of 70 and 220 panel beaters in government and private workshops, respectively. Using a ratio of 1:3, 56 panel beaters from government-owned workshops were selected out of 70 by simple random sampling using the balloting method. From the private-owned workshops, 170 panel beaters were selected. A total of 226 panel beaters were selected from the organized panel beaters considering missed and incomplete responses.
Data and sample were collected using research assistants who were three resident doctors and three phlebotomists. They were trained for two days, two hours per day, on sample collection procedures. Blood for the lead was collected under an aseptic procedure and analyzed using the standard lead stock solution at Project Development Agency (PRODA) Enugu. Blood sample collection was done in an enclosed and well-screened place. The venipuncture system was used to perform venipuncture, and the desired blood of 2–3mls was emptied into Ethylenediaminetetraacetic acid (EDTA) vacutainer bottles for blood lead estimation and complete blood count. The EDTA bottles containing the required amount of blood were gently shaken to
ensure the samples not to be clotted. The samples for lead estimation were transported immediately to the laboratories using the Gio-style cold box after each day, accompanied by 5 and 10ml syringes, bleach, and gloves for maintenance of universal precautions. The blood samples were diluted to 10ml using deionized water because of accompanying cations and anions. The diluted samples were aspirated by the Buck Scientific Model 210 VGP Atomic Absorption Spectrometer (AAS) via a capillary tube at a wavelength of 283.3nm for Pb analysis. The blood samples for complete count were taken to Community Medicine Department Laboratory UNTH Ituku Ozalla for analysis using Mindray Auto Haematology Analyzer. The results of analyses were entered into a proforma or spreadsheet for the blood lead levels and HP.
Data were analyzed using Statistical Package for Social Science (SPSS) version 25. Categorical variables were summarized using frequencies and proportions, while continuous variables were summarized using mean, standard deviation, median, and inter-quartile range for skewed data. The lead levels in blood were categorized using a cut-off of 10µg|dl and above as Pb poisoning [24]. Prevalence of Pb poisoning was noted as a proportion of panel beaters with blood lead of 10µg|dl and above. Haematological parameters were categorized into normal and abnormal using reference values. Comparison of variables was managed using Man Whitney U- (MHU) test, Chi-square test, and T-test. The relationship between blood lead (BPb) and HP was determined using correlation analyses. The level of significance was set at P< 0.05.
Ethical approval was obtained from the Health Research Ethics Committee of the University of Nigeria Teaching Hospital, Ituku Ozalla (No. NHREC/05/01/200BB–FWA00002458–IRB00002323). Permission was obtained from :union:s of roadside and organized panel beaters in Enugu State. Informed consent was obtained from participants.
Results
The median Pb levels were 3.0µg|dl and 16.0µg|dl among roadside and organized panel beaters, respectively. About two-third and one-third of roadside and organized panel beaters, respectively, had Pb level <10µg|dl, while 79 (36.4%) and 138 (63.6%) of roadside and organized panel, respectively, had abnormal levels of Pb. The differences between the two sectors were found to be significant (P< 0.0001) (Table 1).
Table 1. Blood lead estimation among roadside and organized panel beaters
|
Variables |
Roadside panel beaters |
Organized panel beaters |
Statistical analysis |
||||
|
Frequency |
Percent |
Frequency |
Percent |
Test |
P-value |
||
|
Blood lead |
Median |
3.0µg|dl |
- |
16.0µg|dl |
- |
MHU |
<0.0001* |
|
Inter–quartile range |
0 – 20µg|dl |
- |
4 – 31.3µg|dl |
- |
|||
|
Categories of blood lead |
Normal / unexposed |
135 |
64.0 |
76 |
36 |
Chi - square 32.539 |
<0.0001* |
|
Abnormal / poisonous |
79 |
36.4 |
138 |
63.6 |
|||
All the HP on both sectors of panel beaters had mean values within the normal reference values. The mean differences in MCHC, RDWc (%), Neutro (%), Lymph, Lymph (%), Mono (%), and PDWc (%) between the two sectors were found to be significant. Very few respondents among roadside and organized sectors had abnormal HP, 7 (3.3%) of respondents on both sectors had abnormal haemoglobin, 3 (1.4%) of roadside and 12 (5.6%) of organized sector respondents had abnormal RBCs, 4 (1.9%) of roadside and 3 (1.4%) of organized sector respondents had abnormal WBC, and 11 (5.1%) of roadside and 7 (3.3%) of organized sector respondents had abnormal platelets (Tables 2 and 3).
Table 2. Mean distribution of haematogical parameters among roadside and organized panel beaters
|
|
Roadside panel beaters |
Organized panel beaters |
Statistical analysis |
|||
|
Parameters |
Mean |
Std dev. |
Mean |
Std dev. |
T-test |
P-value |
|
Hb (g|dl) |
13.65 |
1.33 |
13.65 |
1.48 |
-0.014 |
0.989 |
|
Haem (%) |
41.43 |
5.02 |
41.93 |
4.56 |
-1.077 |
0.282 |
|
RBC (1012|L) |
5.24 |
2.37 |
5.24 |
0.81 |
0.017 |
0.987 |
|
MCV (fl) |
80.98 |
11.56 |
80.08 |
9.58 |
0.881 |
0.379 |
|
MCH (pg) |
27.41 |
2.86 |
26.60 |
5.46 |
1.909 |
0.057 |
|
MCHC (g|dl) |
32.97 |
1.87 |
32.34 |
2.97 |
2.721 |
0.007* |
|
RDWc (%) |
14.93 |
0.70 |
15.56 |
2.49 |
-3.523 |
<0.001* |
|
RDWSD (fl) |
43.36 |
3.77 |
42.88 |
4.16 |
1.250 |
0.212 |
|
TWBC (109|L) |
5.74 |
1.91 |
6.01 |
1.93 |
-1.443 |
0.150 |
|
Neut. (109|L) |
2.72 |
2.82 |
2.45 |
1.24 |
1.287 |
0.199 |
|
Neut. (%) |
41.53 |
10.53 |
38.88 |
11.84 |
2.447 |
0.015* |
|
Lymph (109|L) |
2.69 |
1.44 |
2.97 |
1.29 |
-2.087 |
0.037* |
|
Lymph (%) |
46.27 |
11.03 |
49.89 |
13.79 |
-3.005 |
0.003* |
|
Monocytes (109|L) |
0.71 |
0.39 |
0.65 |
0.33 |
1.853 |
0.065 |
|
Mono (%) |
11.88 |
4.81 |
10.85 |
3.87 |
2.448 |
0.015* |
|
Platelets (109|L) |
222.30 |
87.72 |
214.81 |
59.63 |
1.034 |
0.302 |
|
MPV (fl) |
8.94 |
0.97 |
9.12 |
1.09 |
-1.839 |
0.067 |
|
PDWc (%) |
15.67 |
0.51 |
20.98 |
10.06 |
-7.719 |
< 0.001* |
|
PCT (%) |
0.20 |
0.09 |
0.19 |
0.07 |
1.300 |
0.194 |
There was a very weak linear relationship between Pb exposure and HP among panel beaters on both sectors, which were mostly negative except for few parameters. The relationship was found not to be significant except for the percentage of neutrophils and monocytes. The scatter plot showed crowding of the haemoglobin, RBC, WBC, and platelet values mostly around the normal values but at lower BPb for roadside panel beaters compared to those of the organized (Table 4, Figs. 1–8).
Table 3. Distribution of haematological parameters among roadside and organized panel beaters
|
|
|
Roadside panel beaters (N = 214) |
Organized panel beaters (N = 214) |
||
|
Parameters |
(Reference values) |
Normal |
Abnormal |
Normal |
Abnormal |
|
Hb (g|dl) |
(10.69 – 18.76) |
207 (96.7) |
7 (3.3) |
207 (96.7) |
7 (3.3) |
|
Haem (%) |
(31.8 – 61.83) |
208 (97.2) |
6 (2.8) |
210 (98.1) |
4 (1.9) |
|
RBC (1012|L) |
(3.61 – 6.97) |
211 (98.6) |
3 (1.4) |
202 (94.4) |
12 (5.6) |
|
MCV (fl) |
(69.71 – 103.23) |
200 (93.5) |
14 (6.5) |
185 (86.4) |
29 (13.6) |
|
MCH (pg) |
(23.30 – 34.16) |
189 (88.3) |
25 (11.7) |
171 (79.9) |
43 (20.1) |
|
MCHC (g|dl) |
(29.74 – 37.16) |
209 (97.7) |
5 (2.3) |
208 (97.2) |
6 (2.8) |
|
RDWCV (%) |
(11.70 – 18.66) |
214 (100) |
0 (0.0) |
211 (98.6) |
3 (1.4) |
|
TWBC (109|L) |
(3.28 – 11.23) |
210 (98.1) |
4 (1.9) |
211 (98.6) |
3 (1.4) |
|
Neut. (109|L) |
(0.65 – 5.50) |
207 (96.7) |
7 (3.3) |
204 (95.3) |
10 (4.7) |
|
Neut. (%) |
(17.57 – 67.46) |
209 (97.7) |
5 (2.3) |
198 (92.5) |
16 (7.5) |
|
Lymph (109|L) |
(0.77 – 4.78) |
211 (98.6) |
3 (1.4) |
206 (96.3) |
8 (3.7) |
|
Lymph (%) |
(11.98 – 66.91) |
207 (96.7) |
7 (3.3) |
187 (87.4) |
27 (12.6) |
|
Mono (109|L) |
(0.21 – 1.02) |
183 (85.5) |
31 (14.5) |
184 (86.0) |
30 (14.0) |
|
Mono (%) |
(4.30 – 15.17) |
170 (79.4) |
44 (20.6) |
189 (88.3) |
25 (11.7) |
|
Platelets (109|L) |
(85.93 – 348.20) |
203 (94.9) |
11 (5.1) |
207 (96.7) |
7 (3.3) |
Table 4. Relationship between estimated lead levels and haematological parameters
|
|
Roadside panel beaters (N = 214) |
Organized panel beaters (N = 214) |
||
|
Parameters |
R |
P-value |
R |
P-value |
|
Hb (g|dl) |
-0.061 |
0.371 |
0.026 |
0.709 |
|
Haem (%) |
-0.090 |
0.191 |
0.016 |
0.821 |
|
RBC (1012|L) |
-0.036 |
0.596 |
-0.004 |
0.958 |
|
MCV (fl) |
-0.005 |
0.948 |
0.034 |
0.616 |
|
MCH (pg) |
-0.062 |
0.368 |
-0.041 |
5.48 |
|
MCHC (g|dl) |
0.039 |
0.570 |
-0.032 |
0.645 |
|
RDWCV (%) |
-0.018 |
0.788 |
-0.064 |
0.349 |
|
RDWSD (fl) |
-0.023 |
0.736 |
0.014 |
0.840 |
|
TWBC (109|L) |
-0.044 |
0.524 |
0.092 |
0.180 |
|
Neut. (109|L) |
-0.008 |
0.909 |
0.096 |
0.162 |
|
Neut. (%) |
0.169 |
0.014 |
0.042 |
0.539 |
|
Lymph (109|L) |
-0.067 |
0.331 |
0.045 |
0.517 |
|
Lymph (%) |
-0.066 |
0.339 |
-0.019 |
0.778 |
|
Monocytes (109|L) |
-0.058 |
0.402 |
-0.021 |
0.761 |
|
Mono (%) |
-0.160 |
0.019 |
-0.092 |
0.181 |
|
Platelets (109|L) |
0.054 |
0.435 |
-0.068 |
0.319 |
|
MPV (fl) |
-0.049 |
0.474 |
-0.108 |
0.116 |
|
PDWc (%) |
-0.002 |
0.972 |
-0.017 |
0.800 |
|
PCT (%) |
0.107 |
0.117 |
-0.072 |
0.294 |


Fig.1. A scatter plot showing the correlation between blood lead levels and haemoglobin among roadside panel beaters


Fig. 2. A scatter plot showing the correlation between blood lead levels and red blood cells among roadside panel beaters
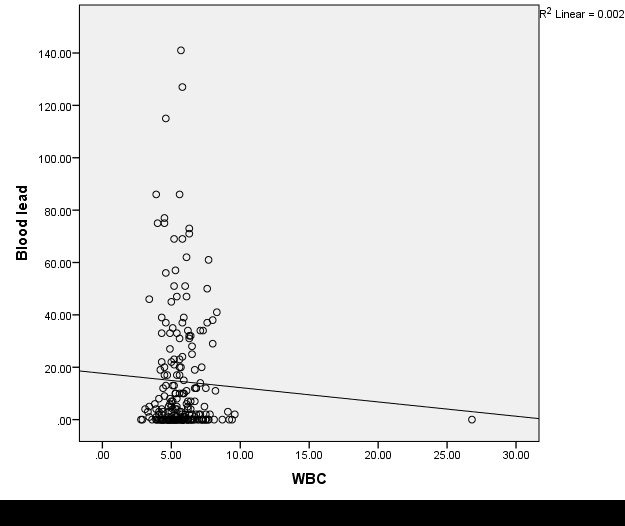

Fig. 3. A scatter plot showing the correlation between blood lead levels and White blood cells among roadside panel beaters


Fig. 4. A scatter plot showing the correlation between blood lead levels and platelets among roadside panel beaters


Fig. 5. A scatter plot showing the correlation between blood lead levels and haemoglobin among organized panel beaters


Fig. 6. A scatter plot showing the correlation between blood lead levels and red blood cells among organized panel beaters


Fig. 7. A scatter plot showing the correlation between blood lead levels and white blood cells among organized panel beaters


Fig. 8. A scatter plot showing the correlation between blood lead levels and platelets among organized panel beaters
Table 5. Parameters and their meanings
|
Parameters |
Definition or full meaning |
|
Hb |
Haemoglobin |
|
Haem (%) |
Haematocrit |
|
RBC |
Red Blood Cells |
|
MCV |
Mean Corpuscular Volume: the average volume of a red blood corpuscle |
|
MCH |
Mean Corpuscular Haemoglobin: the average mass of heamoglobin per red blood cell in a sample of blood |
|
MCHC |
Mean Corpuscular Haemoglobin Concentration: a measure of haemoglobin concentration in a given volume of a packed red blood cell; it is haemoglobin divided by haemotocrit. |
|
RDWCV (%) |
Red Blood Cell Distribution Width: it is a measure of the variation range of red blood cell volume as part of standard complete blood count. |
|
RDWSD |
Red Cell Distribution Width Standard Deviation: it is a measure of the range in the volume and size of red blood cells. |
|
TWBC |
Total White Blood Cells |
|
Neut |
Neutrophils as a component of TWBC |
|
Neut (%) |
Neutrophil as a percentage of TWBC |
|
Lymph |
Lymphocytes |
|
Lymph (%) |
Lymphocytes as a percentage of TWBC |
|
Mono |
Monocytes |
|
Mono (%) |
Monocytes as a percentage of TWBC |
|
Platelets |
Platelets help the blood to clot and stop bleeding. |
|
MPV |
Mean Platelets Volume: the average size of platelets found in the blood |
|
PDWc (%) |
Platelet Distribution Width: it causes activation of coagulation. |
|
PCT (%) |
Platelet Count Test or Plateletcrit: it is the volume occupied by platelets in the blood as a percentage. |
The HP and the respective meanings are described below (Table 5).
Discussion
The haematogical effects of Pb exposure are dose-dependent; notably, the parameters are inhibited at increasing exposure to Pb levels (14, 15). Occupational Pb exposure is considered normal/unexposed at less than 10 µg|dl, above which it becomes poisonous; thus, workers must be moved away from the exposure source (24). The noted effects at the poisonous Pb levels are clinically overt and consequent irreversible health outcomes (24). Following the advent of preventive measures that have halted the burden of Pb toxicities, concerns have shifted to the effects of subclinical toxicities at chronic low doses (4).
Haemoglobin effect from occupational Pb exposure has been widely studied, while few studies exist on effects on other blood cells, mostly in Nigeria compared to other countries. This study assesses all HP and their relationship with Pb levels. The BPb levels in this work show a significant difference in the median BPb of the two sectors. The roadside panel beaters have median BPb within the normal level compared to the organized panel beaters whose median BPb levels are within the abnormal level. The erythrocytic effects of Pb exposure are known to be deranged at Pb levels of 10µg|dl and above and become completed at very high levels (14). The effects on the white blood cells and the platelets are due to the direct toxic action of Pb on lymphoid organs and bone marrow with an increase in total leucocytes, basophils, eosinophils, and lymphocytes, as well as platelet and platelet distribution width (15, 16). In the present research, the mean blood cell values of all the haematological parameters determined on both sectors were found to be within normal references. These values were comparable with the study in Ghana, where haematological values of a healthy population were determined (25). This is because the majority of workers in both sectors have BPb levels within the normal range, and if in the poisoning, they are still within an acceptable range of early onset of hematological effects. The normal mean values of parameters were supported by the majority of workers, >70% in both sectors with HP within the normal reference values. These findings confirmed studies in Nigeria, Jordan, Iraq, and Taiwan with similar Pb levels within an acceptable range (5, 13, 26, 27). However, they differed from studies among children done in Egypt and Iran, where a higher proportion of children were found to have higher mean and abnormal values of HP with BPb within normal values and acceptable range (19, 20, 28). This is due to immature developing bone marrow compared to adults. Lead exposure cause higher direct toxicity in the bone marrow and increase haemolysis of erythrocytic cells. Public health is of concern because of “occupational take-home lead” from clothing, skin, as well as an environmental spillover of Pb particles in the soil, air, and water. Occupational control of personal and environmental hygiene practices is recommended.
The lead exposure on HP found in this study showed a non-significant linear relationship, very weakly correlated in both sectors of panel beaters. Despite linearity, this non-significant correlation is insufficient evidence to predict the relationship between the two variables. The two variables are very close and crowded to zero; however, the slight linearity is potential for toxic outcomes, which should be considered for removal till blood levels are brought to normal or acceptable levels. This is illustrated in the scatter plot in Figs. 1-8, where the HP is crowded within normal and acceptable values of BPb but at lower BPb among the roadside compared to the organized panel beaters. These results are due to the high frequency of normal and acceptable values of estimated Pb levels, chronic nature of exposure to lead, age group of workers, and possibly the nutritional status of workers. It is observed that most workers undertake three nutritious square meals obtainable near their workplace, which are cheaper and adequate. These findings are in line with those of studies in Nigeria, Iran, India, Taiwan, and Italy (5, 7, 13, 16, 29). The findings further show that there could be some uncontrolled Pb exposure with poor haematological outcomes characterized by anaemia, as well as increased leucocytes and platelet aggregation with potential for coagulopathy. This is evident among children in a study in Egypt, where a significant and strong correlation has been found. Good occupational control practices, adequately put in place and adhered to, can help reduce take-home lead, environmental spillover Pb, as well as keeping the haematological parameters within normal levels.
Haematological investigations should be included as part of routine biomonitoring in occupational health practice as an indicator of the health impact of Pb exposure in addition to personal and environmental hygiene practices. The study is specific to one area, and, therefore, the findings cannot be generalized. It is cross-sectional, and the findings are limited to the period of the study.
Conclusion
At normal and acceptable Pb exposures, HP is found to be within normal limits. The few abnormalities are due to few high values of Pb levels. The relationship is found to be non-significant and weakly correlated.
Acknowledgement
The researchers want to express sincere gratitude to subjects who were available for invasive blood collection for this research. We also appreciate the assistance of the Enugu North panel beaters :union:s in reaching to their workers.
Conflict of interest: None declared.
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution 4.0 International License. |








