Volume 11, Issue 1 (Winter 2022)
J Occup Health Epidemiol 2022, 11(1): 1-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemipour E, Vazirinejad R, Sheikh Fatholahi M, Rezaian M. The Survival Rate of Patients with Colorectal Cancer in Kerman Province of Iran from 2007 to 2016. J Occup Health Epidemiol 2022; 11 (1) :1-9
URL: http://johe.rums.ac.ir/article-1-460-en.html
URL: http://johe.rums.ac.ir/article-1-460-en.html
Related article in
Google Scholar
Google Scholar
Similar articles
1- MSc in Epidemiology, Dept. of Epidemiology and Biostatistics, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
2- Professor, Social Determinants of Health Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
3- Assistant Prof., of Biostatistics, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran.
4- Professor, Head of Dept. of Epidemiology and Biostatistics, Occupational Environmental Research Center, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. ,moeygmr2@yahoo.co.uk
2- Professor, Social Determinants of Health Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
3- Assistant Prof., of Biostatistics, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran.
4- Professor, Head of Dept. of Epidemiology and Biostatistics, Occupational Environmental Research Center, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. ,
Article history


Received: 2021/08/15
Accepted: 2022/01/22
ePublished: 2022/03/25
Accepted: 2022/01/22
ePublished: 2022/03/25
Subject:
Epidemiology
Keywords: Colorectal Neoplasms [MeSH], Survival Rate [MeSH], Kaplan–Meier Estimate [MeSH], Proportional Hazards Models [MeSH], Confidence Interval (CI) [MeSH]
Full-Text [PDF 606 kb]
(1052 Downloads)
| Abstract (HTML) (3804 Views)
Table 1. Frequency distribution of the characteristics of patients with CRC based on status (dead, alive) from 2007 to 2016
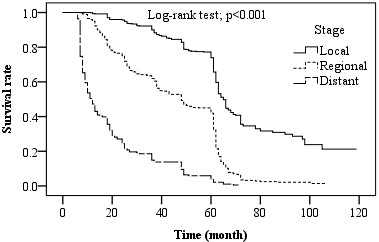
Fig. 1. Five-year survival rate (%) by stages (Local, Regional, Distant)
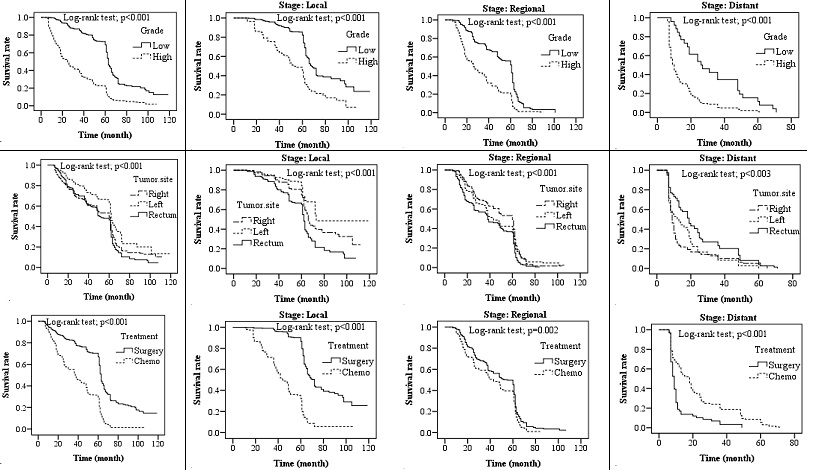
Fig. 2. Kaplan-Meier survival: (A) Overall survival by grade, tumor site, treatment; (B) Survival rate by local stage; (C) Survival rate by regional stage; (D) Survival rate by distant stage
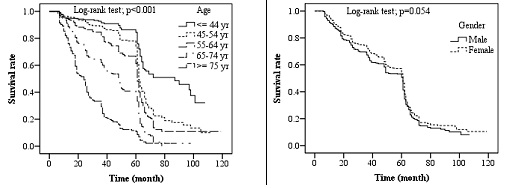
Fig. 3. Kaplan-Meier survival rate by age and sex of patients with colorectal cancer
Table 3. Multivariate analysis of prognostic factors influencing survival of colorectal cancer patients in Cox regression analysis
After determining predictor variables of death in patients with CRC, the interaction between these variables was investigated in the Cox proportional hazards regression model. Statistical analysis results showed that the interaction between predictor variable pairs was not statistically significant. Therefore, only the main effect of predictor variables of death remained in the Cox proportional hazards model, and all interactions between the variable pairs were excluded from the regression model. Time-independent covariates of death in patients with CRC were also evaluated. Statistical analysis showed none of the predictor variables to be time-dependent.
Discussion
This study evaluated the effect of factors potentially associated with the survival rate in CRC patients. The effects of specific variables, including clinical stage, tumor grade, tumor location, and treatment type, on survival rate were evaluated. Given the crucial role of the tumor stage in predicting patient survival, selecting treatment strategies, and evaluating control programs, the effect of each specific factor on patient survival was evaluated separately based on that [13].
The results showed a 5-years survival rate of 51.9% in CRC. Various studies have been conducted worldwide to estimate cancer survival rates. In 2019, WHO reported the results of the global surveillance of cancer survival rates based on the CONCORD3 program [14]. According to these results, CRC survival varied widely in different parts of the world. The highest 5-year survival rate was about 70% in Israel, Jordan, South Korea, and Australia, while the survival was reported less than 50% in Ecuador, Thailand, Russia, and India.
Studies have shown that the CRC survival rate is affected by many factors dependent on patient and tumor characteristics [15]. Differences in CRC survival rates in different countries can be due to differences in access to medical attention and delays in diagnosis and treatment, as well as environmental or lifestyle factors [16]. Also, geographical conditions, as well as cultural, economic, and social factors, influence the distribution of the disease and differences in survival rates [17].
In the present study, the prevalence of CRC was higher in males than females. It should be noted that most studies on the CRC survival rate have shown a higher prevalence in males [18,19]. The mean age of patients in this study was 60.71±15.39 years, and 14.3% were younger than 40 years at the time of diagnosis. In HICs, only 2%- 8% of CRC cases occurred in people under the age of 40 [20,21]. However, the incidence of CRC in the young population is increasing in most LMICs [22]. In addition to numerous indicators related to the development level of the country, environmental factors, and changes in effective behavioral patterns, another major reason for the high incidence rate of CRC among young people in LMICs, including Iran, can be their young age structure [23]. The older age structure in Western countries has made CRC a disease of old age.
This study also evaluated the relationship between tumor-related characteristics and the survival rate. The first specific factor examined was the clinical stage and its effect on survival. According to the results and consistent with previous studies, the clinical stage of the disease significantly affected the 5-year survival rate [24]. Significant changes in 5-year survival rates were observed among different stages of the disease.
Another specific variable was tumor grade. The effect of this factor on the survival rate of patients with CRC was studied. According to the findings, there was a significant difference between the survival rate of patients with high-grade tumors and those with low-grade tumors. These differences were evident at all stages of the disease, being confirmed by numerous studies [25].
The relationship between tumor location and survival rate was also evaluated, which showed the overall survival in patients with LCC to be better than that in those with RCC and RECC [26]. After examining the effect of tumor location on survival, different behaviors were observed in regional and distant stages. Patients with RCC in the regional stage and those with RECC in the distant stage showed higher survival rates.
Patients undergoing surgery had a better survival rate than patients undergoing chemotherapy, except for those in the distant stage [27]. This difference can be explained by the fact that the type of treatment is chosen based on the disease stage in CRC. Chemotherapy is the treatment of choice in the distant stage, in which patients have clearly a lower survival rate due to the disease spread, while surgery is the treatment of choice for the local stage and many cases in the regional stage. In these two stages, especially that of local, patients have a higher survival rate. Another reason is the influence of age on treatment choice. Younger patients are more suitable candidates for surgical treatment compared to others, while surgery is selected for older patients cautiously [28]. In line with previous studies, the present study showed that the survival rate significantly decreased in all diagnostic stages by age [29,30].
In this study, females had a higher survival rate than males, and males had a higher risk of dying from colon and rectal cancer than females. This finding is supported by many studies that have evaluated the effect of sex on the CRC survival rate [31]. It can be attributed to the social support and greater acceptance of health-centered programs in females and the negative attitude toward screening programs and less attention to medical advice in males. Other factors may also influence this difference, thus requiring further studies.
In this study, specific and diagnostic data and demographic characteristics of patients with CRC were used as the information source to estimate survival rates. It is worth noting that even though this study was based on a large population, it had potential limitations. First, despite careful review of the measurements at cancer registry centers to prevent inaccurate data recording, as well as data re-evaluation at the Central Cancer Registry for accuracy and quality, a few cases of data miscoding were observed. These discrepancies were largely resolved by obtaining reports from the diagnosis and pathology centers where the patients were registered. Second, studied cancer registration systems contained treatment information on surgery and chemotherapy, but adjuvant treatment information was not available.
The results can also be used to devise effective strategies for CRC screening programs and develop effective educational programs to raise public awareness about disease symptoms and the importance of participating in screening programs for early detection.
Conclusion
Using population-based cancer registry information, we analyzed data from patients with CRC and estimated their survival rates according to the clinical stage. The overall 5-years survival rate was 51.9%. The tumor stage at diagnosis, as the most important determinant of survival, was significantly correlated with the survival rate of patients; this rate was significantly higher in the local stage patients than in others. Consistent with our findings, studies on CRC survival rates worldwide have reported a statistically significant relationship between the tumor stage and the survival rate. In the present study, increased age, low tumor differentiation, distant stage of the tumor, and RCC were associated with lower survival. CRC is preventable cancer, and patients’ survival rate increases significantly with the diagnosis of the tumor in an early stage. Despite advances in the diagnosis and treatment of CRC in Iran and worldwide, the 5-year survival rate of patients in this study was lower than that of HICs. Since many factors affect this rate, further studies are required to identify prognostic factors of survival rate. The implementation of targeted screening strategies is also essential for the timely diagnosis and treatment of patients to improve colorectal cancer survival rates in the Iranian population.
Acknowledgement
The authors would like to thank Kerman, Rafsanjan, Jiroft, and Bam academic cancer registry centers for providing the relevant data.
Conflict of interest: None declared.
Full-Text: (613 Views)
Introduction
Cancer is a major public health problem throughout the world. In recent years, it has become the second leading cause of death among chronic diseases in high-income countries (HICs) after cardiovascular disease; further, epidemiological evidence reveals a similar trend in low- and middle-income countries (LMICs) [1]. Of the long list of cancers, colorectal cancer (CRC) is among the most common worldwide [2]. CRC is the third common cancer in males after lung and prostate cancers and the second common cancer in females after breast cancer [3]. It is also the fourth and third leading cause of cancer deaths in males and females globally, respectively [4]. In 2019 worldwide, CRC is also considered the second leading cause of cancer disability-adjusted life years (DALYs) [5].
Evidence suggests that incident cases of CRC are rising swiftly in LMICs due to the increased prevalence of changeable risk factors, including alcohol drinking, smoking, unhealthy eating behaviors, obesity, and sedentary lifestyle [1]. According to the latest statistics and epidemiological studies in Iran, CRC is the fourth common cancer in males and the second in females, as well as the fourth and third leading cause of cancer deaths in males and females, respectively [6].
Survival rates for CRC vary widely across the world [7]. Studies have shown that the survival rate in patients with CRC depends not only on the anatomical extent of the disease but also on many patient- and tumor-related factors [8]. Given the preventable nature of CRC, proper planning is essential to prevent and control this cancer [9]. Also, its timely diagnosis in the pre-symptomatic stage plays a vital role in improving the quality of life and survival rate in patients [10].
Kerman is the largest province of Iran located in the south. In this province, CRC is the fifth common cancer [11]. However, despite this relatively high prevalence, detailed statistics are not available on the survival rate in CRC patients in the province. Since the first principle in the prevention and control of CRC is to know its conditions at the local level, this study is carried out to examine the 5-year survival rate of CRC patients in Kerman Province. It is hoped that the current study results provide a basis for promoting CRC prevention and control programs in the province.
Materials and Methods
In this survival analysis study, data on all patients with CRC were collected using a checklist from four academic cancer registry centers in the provinces, including Kerman, Rafsanjan, Jiroft, and Bam. In the next step, extracted data for 1705 patients with a definite diagnosis of CRC, registered at those cancer registry centers, were examined from March 21, 2007, up to March 20, 2017, for their survival status. Mortality data of the patients, including the exact date and cause of death, were examined annually. Patients with uncertain death or survival status, as well as those who died from causes other than CRC (N=38), were excluded from further analyses. At the final
stage, data, including age, gender, tumor location, degree of tumor differentiation, stage of the disease at the time of diagnosis, treatment modality (surgery/chemotherapy), and patient death, were used in the analysis.
Based on extracted data, the stage of the disease at diagnosis was classified as local (tumor confined to the primary site), regional (tumor expanded to adjacent tissues and/or to regional or distant lymph nodes), and distant (tumor spread to distant organs). Tumor location was divided into right-sided colon cancer (RCC), left-sided colon cancer (LCC), and rectal cancer (RECC). RCC includes parts from the cecum to the transverse colon. LCC comprises the splenic flexure to the sigmoid. Finally, RECC consists of the rectum, rectosigmoid, anus, anal canal, and anorectal area. The degree of tumor differentiation was classified as low-grade (well- and moderately-differentiated) and high-grade (poorly differentiated /anaplastic) [9].
For analyzing the data, the chi-square test was used to determine the relationship of each factor with the patient status (death, survival). The Kaplan–Meier estimator was used to estimate the survival rate and plot its curves. The Log-rank test was utilized to assess differences in the survival rate among the groups studied. Given that the tumor staging system is the proper guide for predicting disease progression and making decisions about therapeutic strategies in patients with cancer [12], the relationship of each tumor-specific variable with patient survival was assessed based on the diagnostic stage of the disease. Finally, univariate analysis and Cox proportional-hazards model were used to determine relationships between CRC-related factors and patient survival. Data were analyzed using standardized methods in SAS-9.1 software. The significance level was set at P<0.05.
Ethical approval was obtained from the Rafsanjan University of Medical Sciences Ethics Committee (No. IR.RUMS.REC.1396.189).
Results
In this study, 929 patients (54.5%) were male and 776 (45.5%) female. The mean and standard deviation of patients’ age was 60.71±15.39 years (range: 10-100 years). Of them, 916 (53.7%) died, and 789 (46.3%) were still alive by the end of this study. Table 1 provides the frequency distribution of patients’ characteristics based on their status (dead, alive).
Cancer is a major public health problem throughout the world. In recent years, it has become the second leading cause of death among chronic diseases in high-income countries (HICs) after cardiovascular disease; further, epidemiological evidence reveals a similar trend in low- and middle-income countries (LMICs) [1]. Of the long list of cancers, colorectal cancer (CRC) is among the most common worldwide [2]. CRC is the third common cancer in males after lung and prostate cancers and the second common cancer in females after breast cancer [3]. It is also the fourth and third leading cause of cancer deaths in males and females globally, respectively [4]. In 2019 worldwide, CRC is also considered the second leading cause of cancer disability-adjusted life years (DALYs) [5].
Evidence suggests that incident cases of CRC are rising swiftly in LMICs due to the increased prevalence of changeable risk factors, including alcohol drinking, smoking, unhealthy eating behaviors, obesity, and sedentary lifestyle [1]. According to the latest statistics and epidemiological studies in Iran, CRC is the fourth common cancer in males and the second in females, as well as the fourth and third leading cause of cancer deaths in males and females, respectively [6].
Survival rates for CRC vary widely across the world [7]. Studies have shown that the survival rate in patients with CRC depends not only on the anatomical extent of the disease but also on many patient- and tumor-related factors [8]. Given the preventable nature of CRC, proper planning is essential to prevent and control this cancer [9]. Also, its timely diagnosis in the pre-symptomatic stage plays a vital role in improving the quality of life and survival rate in patients [10].
Kerman is the largest province of Iran located in the south. In this province, CRC is the fifth common cancer [11]. However, despite this relatively high prevalence, detailed statistics are not available on the survival rate in CRC patients in the province. Since the first principle in the prevention and control of CRC is to know its conditions at the local level, this study is carried out to examine the 5-year survival rate of CRC patients in Kerman Province. It is hoped that the current study results provide a basis for promoting CRC prevention and control programs in the province.
Materials and Methods
In this survival analysis study, data on all patients with CRC were collected using a checklist from four academic cancer registry centers in the provinces, including Kerman, Rafsanjan, Jiroft, and Bam. In the next step, extracted data for 1705 patients with a definite diagnosis of CRC, registered at those cancer registry centers, were examined from March 21, 2007, up to March 20, 2017, for their survival status. Mortality data of the patients, including the exact date and cause of death, were examined annually. Patients with uncertain death or survival status, as well as those who died from causes other than CRC (N=38), were excluded from further analyses. At the final
stage, data, including age, gender, tumor location, degree of tumor differentiation, stage of the disease at the time of diagnosis, treatment modality (surgery/chemotherapy), and patient death, were used in the analysis.
Based on extracted data, the stage of the disease at diagnosis was classified as local (tumor confined to the primary site), regional (tumor expanded to adjacent tissues and/or to regional or distant lymph nodes), and distant (tumor spread to distant organs). Tumor location was divided into right-sided colon cancer (RCC), left-sided colon cancer (LCC), and rectal cancer (RECC). RCC includes parts from the cecum to the transverse colon. LCC comprises the splenic flexure to the sigmoid. Finally, RECC consists of the rectum, rectosigmoid, anus, anal canal, and anorectal area. The degree of tumor differentiation was classified as low-grade (well- and moderately-differentiated) and high-grade (poorly differentiated /anaplastic) [9].
For analyzing the data, the chi-square test was used to determine the relationship of each factor with the patient status (death, survival). The Kaplan–Meier estimator was used to estimate the survival rate and plot its curves. The Log-rank test was utilized to assess differences in the survival rate among the groups studied. Given that the tumor staging system is the proper guide for predicting disease progression and making decisions about therapeutic strategies in patients with cancer [12], the relationship of each tumor-specific variable with patient survival was assessed based on the diagnostic stage of the disease. Finally, univariate analysis and Cox proportional-hazards model were used to determine relationships between CRC-related factors and patient survival. Data were analyzed using standardized methods in SAS-9.1 software. The significance level was set at P<0.05.
Ethical approval was obtained from the Rafsanjan University of Medical Sciences Ethics Committee (No. IR.RUMS.REC.1396.189).
Results
In this study, 929 patients (54.5%) were male and 776 (45.5%) female. The mean and standard deviation of patients’ age was 60.71±15.39 years (range: 10-100 years). Of them, 916 (53.7%) died, and 789 (46.3%) were still alive by the end of this study. Table 1 provides the frequency distribution of patients’ characteristics based on their status (dead, alive).
Table 1. Frequency distribution of the characteristics of patients with CRC based on status (dead, alive) from 2007 to 2016
| P-value | df | χ2 | All (n=1705) |
Alive (n=789) | Dead (n=916) |
Variable | |
| <0.001 | 2 | 312.077 | 832(48.8) | 549(69.6) | 283(30.9) | Local | Stage |
| 677(39.7) | 233(29.5) | 444(48.5) | Regional | ||||
| 196(11.5) | 7(0.9) | 189(20.6) | Distant | ||||
| <0.001 | 2 | 90.096 | 528(31.0) | 198(25.1) | 330(36.0) | Right colon | Tumor location |
| 529(31.0) | 336(42.6) | 193(21.1) | Left colon | ||||
| 648(38.0) | 255(32.3) | 393(42.9) | Rectal | ||||
| <0.001 | 2 | 279.870 | 1005(58.9) | 632(82.2) | 373(48.1) | Low grade | Grade |
| 540(31.7) | 137(17.8) | 403(51.9) | High grade | ||||
| 160(9.4) | 20(2.5) | 140(15.3) | Unknown | ||||
| <0.001 | 1 | 140.544 | 1072(62.9) | 614(77.8) | 458(50.0) | Surgery | Treatment |
| 633(37.1) | 175(22.2) | 458(50.0) | Chemotherapy | ||||
| <0.001 | 4 | 112.787 | 244(14.3) | 170(21.5) | 74(8.1) | 45> | Age |
| 301(17.7) | 159(20.2) | 142(15.5) | 45-54 | ||||
| 425(24.9) | 207(26.2) | 218(23.8) | 55-64 | ||||
| 399(23.4) | 160(20.3) | 239(26.1) | 65-74 | ||||
| 336(19.7) | 93(11.8) | 243(26.5) | 75≤ | ||||
| 0.565 | 1 | 0.331 | 929(54.5) | 424(53.7) | 505(55.1) | Male | Sex |
| 776(45.5) | 365(46.3) | 411(44.9) | Female |
According to the chi-square test, diagnostic stage, tumor location, tumor grade, treatment type, and the age group showed a significant relationship with patient status (dead, alive) (p<0.001).
Survival rates: In this study, the median 5-year
survival for patients with CRC was 61.0 months, and the overall survival rate was 51.9%. Table 2 presents 0- to 5-year survival rates based on tumor stage, tumor grade, tumor location, treatment type, age, and sex.
Survival rates: In this study, the median 5-year
survival for patients with CRC was 61.0 months, and the overall survival rate was 51.9%. Table 2 presents 0- to 5-year survival rates based on tumor stage, tumor grade, tumor location, treatment type, age, and sex.
Table 2. Five-year survival (%) by prognostic factors for patients with colorectal cancer
| P-value | Median Survival (month) | 60 months | 48 months | 36 months | 24 months | 12 months | Variable | |
| <.001 | 65.0 | 73.9 | 82.8 | 90.0 | 95.7 | 99.3 | Local | Stage |
| 48.0 | 41.9 | 49.3 | 62.0 | 75.1 | 95.1 | Regional | ||
| 12.0 | 3.7 | 9.6 | 14.9 | 24.4 | 47.3 | Distant | ||
| <.001 | 63.0 | 69.0 | 76.9 | 85.4 | 92.6 | 99.2 | Low | Grade |
| 25.0 | 20.2 | 27.8 | 37.8 | 51.1 | 75.1 | High | ||
| <.001 | 60.0 | 49.1 | 57.0 | 65.0 | 75.0 | 86.9 | RCC | Tumor location |
| 62.0 | 62.2 | 68.9 | 76.9 | 85.0 | 94.9 | LCC | ||
| 51.0 | 46.4 | 55.7 | 67.2 | 76.8 | 92.2 | RECC | ||
| <.001 | 63.0 | 66.9 | 74.0 | 80.9 | 86.7 | 93.9 | Surgery | Treatment |
| 37.0 | 28.8 | 38.5 | 51.5 | 66.0 | 87.3 | Chemotherapy | ||
| <.001 | 87.0 | 84.4 | 89.6 | 91.5 | 93.8 | 96.4 | <45 | Age |
| 62.0 | 73.6 | 82.5 | 88.9 | 92.4 | 96.2 | 45-54 | ||
| 61.0 | 61.2 | 70.2 | 81.4 | 87.3 | 94.7 | 55-64 | ||
| 49.0 | 38.1 | 50.3 | 61.9 | 74.3 | 91.1 | 65-74 | ||
| 24.0 | 10.9 | 16.1 | 29.4 | 42.9 | 80.4 | ≥75 | ||
| .054 | 61.0 | 50.7 | 58.4 | 67.3 | 76.6 | 90.0 | Male | Sex |
| 61.0 | 53.3 | 62.3 | 72.3 | 81.5 | 93.1 | Female |
Survival rate based on tumor stage: The survival rate in patients with CRC was evaluated using the tumor staging system. The 5-year survival rate in the local, regional, and distant stages was 73.9%, 41.9%, and 3.7%, respectively. Comparison of survival rates showed a significant difference between stages of the disease (p<0.001) (Fig. 1), such that the survival rate was significantly higher in the local stage than that in regional and distant stages (p<0.001). Also, the survival rate in the regional stage was significantly higher than that in the distant stage (p<0.001).

Fig. 1. Five-year survival rate (%) by stages (Local, Regional, Distant)
Survival rate based on tumor grade: The Log-rank test showed a statistically significant difference in the survival rate in patients during the follow-up period based on the tumor grade (p<0.001). Survival was higher in low-grade tumors than that in high-grade ones. Survival was then evaluated for tumor grade based on defined clinical stages. Survival curves in all three stages of CRC showed higher survival in low-grade than that in high-grade tumors (Fig. 2).
Survival rate based on tumor location: Survival curves showed a significant correlation between the survival rate and tumor location (Fig. 2). The survival rate of patients with LCC showed a statistically significant difference from those of cases with RCC and RECC (p<0.001). Generally, LCC had a higher survival rate than RCC and RECC (p<0.001). There was no statistical difference between the survival rates of RCC and RECC. In the next step, the survival rate in patients was estimated based on tumor location in terms of the clinical stage, where survival curves at each stage showed different trends. For instance, in the local stage, the survival rate in LCC was higher than that in RCC (p<0.001). Also, the survival rate in RCC was significantly higher than that in RECC (p<0.001).
Survival rate based on the treatment type: Survival curves showed a significant correlation between the survival rate in patients with CRC and their treatment type (Fig. 2). There was a statistically significant difference in the survival rate between patients treated by surgery and those treated by chemotherapy (p<0.001); those treated by surgery had a higher survival rate. In the next step, the survival rate of patients was estimated based on the treatment type in terms of the clinical stage, where survival curves at different stages showed different trends. The survival rate in the distant stage was significantly higher in patients undergoing chemotherapy than that in those undergoing surgery (p<0.001).
Survival rate based on age groups and sex: Patients in this study were divided into five age groups of <45, 45-54, 55-64, 65-74, and ≥75 years. Survival curves by age groups showed a significant difference in 5-year survival rates. The highest survival rate was that of the <45 years age group, which decreased by age, and the lowest survival rate was that of the age group ≥75 years (p<0.001). In survival rate evaluation based on sex, survival curves did not show a significant difference (p=0.054). Fig. 3 shows the survival curves of age and sex variables.
Survival rate based on tumor location: Survival curves showed a significant correlation between the survival rate and tumor location (Fig. 2). The survival rate of patients with LCC showed a statistically significant difference from those of cases with RCC and RECC (p<0.001). Generally, LCC had a higher survival rate than RCC and RECC (p<0.001). There was no statistical difference between the survival rates of RCC and RECC. In the next step, the survival rate in patients was estimated based on tumor location in terms of the clinical stage, where survival curves at each stage showed different trends. For instance, in the local stage, the survival rate in LCC was higher than that in RCC (p<0.001). Also, the survival rate in RCC was significantly higher than that in RECC (p<0.001).
Survival rate based on the treatment type: Survival curves showed a significant correlation between the survival rate in patients with CRC and their treatment type (Fig. 2). There was a statistically significant difference in the survival rate between patients treated by surgery and those treated by chemotherapy (p<0.001); those treated by surgery had a higher survival rate. In the next step, the survival rate of patients was estimated based on the treatment type in terms of the clinical stage, where survival curves at different stages showed different trends. The survival rate in the distant stage was significantly higher in patients undergoing chemotherapy than that in those undergoing surgery (p<0.001).
Survival rate based on age groups and sex: Patients in this study were divided into five age groups of <45, 45-54, 55-64, 65-74, and ≥75 years. Survival curves by age groups showed a significant difference in 5-year survival rates. The highest survival rate was that of the <45 years age group, which decreased by age, and the lowest survival rate was that of the age group ≥75 years (p<0.001). In survival rate evaluation based on sex, survival curves did not show a significant difference (p=0.054). Fig. 3 shows the survival curves of age and sex variables.

Fig. 2. Kaplan-Meier survival: (A) Overall survival by grade, tumor site, treatment; (B) Survival rate by local stage; (C) Survival rate by regional stage; (D) Survival rate by distant stage

Fig. 3. Kaplan-Meier survival rate by age and sex of patients with colorectal cancer
Finally, the correlation of each variable with the survival rate of CRC patients was evaluated through Cox proportional hazards regression model (Table 3). In the Adjusted Regression Model, the risk increased significantly with age. The risk of death in the age group of ≥75 years was 7.660 times higher than that in the age group of <45 years (p<0.0001). Moreover, the risk of death in female patients with CRC was lower than that in males (Hazard Ratio; HR=0.689, p<0.0001).
The risk of death in patients with high tumor grades was 3.390 times higher than that in patients with low tumor grades (p<0.0001). Type of treatment, after adjustments in the model, showed no significant difference in the risk level; however, the survival rate in patients undergoing chemotherapy was lower than that in patients treated by surgery (HR = 1.081, p= 0.3637).
While the risk was not significantly different for different tumor locations, there was a higher survival rate in patients with tumors in LCC (HR = 0.849, p= 0.1053). Furthermore, based on the tumor stage, the risk was 3.147 times higher in the regional stage and 9.412 times higher in the distant stage than that in the local stage (p<0.0001).
The risk of death in patients with high tumor grades was 3.390 times higher than that in patients with low tumor grades (p<0.0001). Type of treatment, after adjustments in the model, showed no significant difference in the risk level; however, the survival rate in patients undergoing chemotherapy was lower than that in patients treated by surgery (HR = 1.081, p= 0.3637).
While the risk was not significantly different for different tumor locations, there was a higher survival rate in patients with tumors in LCC (HR = 0.849, p= 0.1053). Furthermore, based on the tumor stage, the risk was 3.147 times higher in the regional stage and 9.412 times higher in the distant stage than that in the local stage (p<0.0001).
Table 3. Multivariate analysis of prognostic factors influencing survival of colorectal cancer patients in Cox regression analysis
| P-value | Adjusted hazard ratio (95% CI) | Adjusted hazard ratio | P-value | Unadjusted hazard ratio (95% CI) | Unadjusted hazard ratio | Variable | |
| - | - | 1(Ref) | - | - | 1(Ref) | 45> | Age |
| .0016 | 1.250-2.613 | 1.808 | <.0001 | 1.461-2.565 | 1.936 | 45-54 | |
| <.0001 | 1.514-3.210 | 2.212 | <.0001 | 2.005-3.411 | 2.615 | 55-64 | |
| <.0001 | 2.484-5.690 | 3.760 | <.0001 | 3.579-6.088 | 4.668 | 65-74 | |
| <.0001 | 4.854-12.087 | 7.660 | <.0001 | 8.621-14.881 | 11.327 | 75≤ | |
| - | - | 1(Ref) | - | - | 1(Ref) | Male | Sex |
| .0001 | 0.570-0.835 | 0.689 | .0552 | 0.772-1.003 | 0.880 | Female | |
| - | - | 1(Ref) | - | - | 1(Ref) | Low grade | Grade |
| <.0001 | 2.878-3.994 | 3.390 | <.0001 | 3.360-4.474 | 3.877 | High grade | |
| - | - | 1(Ref) | - | - | 1(Ref) | Surgery | Treatment |
| .3637 | 0.914-1.277 | 1.081 | <.0001 | 2.546-3.330 | 2.912 | Chemotherapy | |
| - | - | 1(Ref) | - | - | 1(Ref) | RCC | Tumor location |
| .1053 | 0.697-1.035 | 0.849 | <.0001 | 0.573-0.820 | 0.658 | LCC | |
| .1942 | 0.944-1.330 | 1.120 | .0848 | 0.982-1.319 | 1.138 | RECC | |
| - | - | 1(Ref) | - | - | 1(Ref) | Local | |
| <.0001 | 2.637-3.756 | 3.147 | <.0001 | 2.700-3.656 | 3.142 | Regional | Stage |
| <.0001 | 7.387-11.993 | 9.412 | <.0001 | 10.688-15.734 | 12.955 | Distant |
After determining predictor variables of death in patients with CRC, the interaction between these variables was investigated in the Cox proportional hazards regression model. Statistical analysis results showed that the interaction between predictor variable pairs was not statistically significant. Therefore, only the main effect of predictor variables of death remained in the Cox proportional hazards model, and all interactions between the variable pairs were excluded from the regression model. Time-independent covariates of death in patients with CRC were also evaluated. Statistical analysis showed none of the predictor variables to be time-dependent.
Discussion
This study evaluated the effect of factors potentially associated with the survival rate in CRC patients. The effects of specific variables, including clinical stage, tumor grade, tumor location, and treatment type, on survival rate were evaluated. Given the crucial role of the tumor stage in predicting patient survival, selecting treatment strategies, and evaluating control programs, the effect of each specific factor on patient survival was evaluated separately based on that [13].
The results showed a 5-years survival rate of 51.9% in CRC. Various studies have been conducted worldwide to estimate cancer survival rates. In 2019, WHO reported the results of the global surveillance of cancer survival rates based on the CONCORD3 program [14]. According to these results, CRC survival varied widely in different parts of the world. The highest 5-year survival rate was about 70% in Israel, Jordan, South Korea, and Australia, while the survival was reported less than 50% in Ecuador, Thailand, Russia, and India.
Studies have shown that the CRC survival rate is affected by many factors dependent on patient and tumor characteristics [15]. Differences in CRC survival rates in different countries can be due to differences in access to medical attention and delays in diagnosis and treatment, as well as environmental or lifestyle factors [16]. Also, geographical conditions, as well as cultural, economic, and social factors, influence the distribution of the disease and differences in survival rates [17].
In the present study, the prevalence of CRC was higher in males than females. It should be noted that most studies on the CRC survival rate have shown a higher prevalence in males [18,19]. The mean age of patients in this study was 60.71±15.39 years, and 14.3% were younger than 40 years at the time of diagnosis. In HICs, only 2%- 8% of CRC cases occurred in people under the age of 40 [20,21]. However, the incidence of CRC in the young population is increasing in most LMICs [22]. In addition to numerous indicators related to the development level of the country, environmental factors, and changes in effective behavioral patterns, another major reason for the high incidence rate of CRC among young people in LMICs, including Iran, can be their young age structure [23]. The older age structure in Western countries has made CRC a disease of old age.
This study also evaluated the relationship between tumor-related characteristics and the survival rate. The first specific factor examined was the clinical stage and its effect on survival. According to the results and consistent with previous studies, the clinical stage of the disease significantly affected the 5-year survival rate [24]. Significant changes in 5-year survival rates were observed among different stages of the disease.
Another specific variable was tumor grade. The effect of this factor on the survival rate of patients with CRC was studied. According to the findings, there was a significant difference between the survival rate of patients with high-grade tumors and those with low-grade tumors. These differences were evident at all stages of the disease, being confirmed by numerous studies [25].
The relationship between tumor location and survival rate was also evaluated, which showed the overall survival in patients with LCC to be better than that in those with RCC and RECC [26]. After examining the effect of tumor location on survival, different behaviors were observed in regional and distant stages. Patients with RCC in the regional stage and those with RECC in the distant stage showed higher survival rates.
Patients undergoing surgery had a better survival rate than patients undergoing chemotherapy, except for those in the distant stage [27]. This difference can be explained by the fact that the type of treatment is chosen based on the disease stage in CRC. Chemotherapy is the treatment of choice in the distant stage, in which patients have clearly a lower survival rate due to the disease spread, while surgery is the treatment of choice for the local stage and many cases in the regional stage. In these two stages, especially that of local, patients have a higher survival rate. Another reason is the influence of age on treatment choice. Younger patients are more suitable candidates for surgical treatment compared to others, while surgery is selected for older patients cautiously [28]. In line with previous studies, the present study showed that the survival rate significantly decreased in all diagnostic stages by age [29,30].
In this study, females had a higher survival rate than males, and males had a higher risk of dying from colon and rectal cancer than females. This finding is supported by many studies that have evaluated the effect of sex on the CRC survival rate [31]. It can be attributed to the social support and greater acceptance of health-centered programs in females and the negative attitude toward screening programs and less attention to medical advice in males. Other factors may also influence this difference, thus requiring further studies.
In this study, specific and diagnostic data and demographic characteristics of patients with CRC were used as the information source to estimate survival rates. It is worth noting that even though this study was based on a large population, it had potential limitations. First, despite careful review of the measurements at cancer registry centers to prevent inaccurate data recording, as well as data re-evaluation at the Central Cancer Registry for accuracy and quality, a few cases of data miscoding were observed. These discrepancies were largely resolved by obtaining reports from the diagnosis and pathology centers where the patients were registered. Second, studied cancer registration systems contained treatment information on surgery and chemotherapy, but adjuvant treatment information was not available.
The results can also be used to devise effective strategies for CRC screening programs and develop effective educational programs to raise public awareness about disease symptoms and the importance of participating in screening programs for early detection.
Conclusion
Using population-based cancer registry information, we analyzed data from patients with CRC and estimated their survival rates according to the clinical stage. The overall 5-years survival rate was 51.9%. The tumor stage at diagnosis, as the most important determinant of survival, was significantly correlated with the survival rate of patients; this rate was significantly higher in the local stage patients than in others. Consistent with our findings, studies on CRC survival rates worldwide have reported a statistically significant relationship between the tumor stage and the survival rate. In the present study, increased age, low tumor differentiation, distant stage of the tumor, and RCC were associated with lower survival. CRC is preventable cancer, and patients’ survival rate increases significantly with the diagnosis of the tumor in an early stage. Despite advances in the diagnosis and treatment of CRC in Iran and worldwide, the 5-year survival rate of patients in this study was lower than that of HICs. Since many factors affect this rate, further studies are required to identify prognostic factors of survival rate. The implementation of targeted screening strategies is also essential for the timely diagnosis and treatment of patients to improve colorectal cancer survival rates in the Iranian population.
Acknowledgement
The authors would like to thank Kerman, Rafsanjan, Jiroft, and Bam academic cancer registry centers for providing the relevant data.
Conflict of interest: None declared.
References
1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66(4):683-91. [DOI] [PMID]
2. Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018; 124(13):2785-800. [DOI] [PMID] [PMCID]
3. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70(3):145-64. [DOI] [PMID]
4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359-86. [DOI] [PMID]
5. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and 417 territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet 2020; 396(10258):1204-22. [DOI]
6. Roshandel G, Ghanbari-Motlagh A, Partovipour E, Salavati F, Hasanpour-Heidari S, Mohammadi G, et al. Cancer incidence in Iran in 2014: Results of the Iranian National Population-based Cancer Registry. Cancer Epidemiol 2019; 61:50-8. [DOI] [PMID]
7. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69(5):363-85. [DOI] [PMID]
8. Jeffery M, Hickey BE, Hider PN. Follow‐up strategies for patients treated for non‐metastatic colorectal cancer. Cochrane Database Syst Rev 2019; 9(9):CD002200. [DOI] [PMID] [PMCID]
9. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(23):2564-75. [DOI] [PMID]
10. Ahmed S, Leis A, Fields A, Chandra-Kanthan S, Haider K, Alvi R, et al. Survival Impact of Surgical Resection of Primary Tumor in Patients With Stage IV Colorectal Cancer: Results From a Large Population-Based Cohort Study. Cancer 2014; 120(5):683-91. [DOI] [PMID]
11. Keyghobadi N, Rafiemanesh H, Mohammadian A, Enayatrad M, Salehiniya H. Epidemiology and Trend of Cancers in the Province of Kerman: Southeast of Iran. Asian Pac J Cancer Prev 2015; 16(4):1409-13. [DOI] [PMID]
12. Karim S, Brennan K, Nanji S, Berry SR, Booth CM. Association between Prognosis and Tumor Laterality in Early-Stage Colon Cancer. JAMA Oncol 2017; 3(10):1386-92. [DOI] [PMID] [PMCID]
13. Liang WY, Wang YC, Hsu CY, Yang SH. Staging of colorectal cancers based on elastic lamina invasion. Hum Pathol 2019; 85:44-9. [] [PMID]
14. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391(10125):1023-75. [DOI] [PMID] [PMCID]
15. Engel C, Vasen HF, Seppälä T, Aretz S, Bigirwamungu-Bargeman M, de Boer SY, et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy among 3 Countries with Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018; 155(5):1400-9. [DOI] [PMID]
16. Carr PR, Weigl K, Jansen L, Walter V, Erben V, Chang-Claude J, et al. Healthy Lifestyle Factors Associated with Lower Risk of Colorectal Cancer Irrespective of Genetic Risk. Gastroenterology 2018; 155(6):1805-15. [DOI] [PMID] [PMCID]
17. Wang X, Mao M, Xu G, Lin F, Sun P, Baklaushev VP, et al. The incidence, associated factors, and predictive nomogram for early death in stage IV colorectal cancer. Int J Colorectal Dis 2019; 34(7):1189-201. [DOI] [PMID]
18. Rim CH, Kim CY, Yang DS, Yoon WS. Clinical Significance of Gender and Body Mass Index in Asian Patients with Colorectal Cancer. J Cancer 2019; 10(3):682-8. [DOI] [PMID] [PMCID]
19. Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med 2018; 168(11):775-82. [DOI] [PMID] [PMCID]
20. Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018; 124(14):2964-73. [DOI] [PMID] [PMCID]
21. Hayes L, Forrest L, Adams J, Hidajat M, Ben-Shlomo Y, White M, et al. Age-related inequalities in colon cancer treatment persist over time: a population-based analysis. J Epidemiol Community Health 2019; 73(1):34-41. [DOI] [PMID]
22. Sung JJY, Chiu HM, Jung KW, Jun JK, Sekiguchi M, Matsuda T, et al. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol 2019; 114(2):322-9. [DOI] [PMID]
23. Ansa BE, Coughlin SS, Alema-Mensah E, Smith SA. Evaluation of Colorectal Cancer Incidence Trends in the United States (2000–2014). J Clin Med 2018; 7(2):22. [DOI] [PMID] [PMCID]
24. McSorley ST, Black DH, Horgan PG, McMillan DC. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr 2018; 37(4):1279-85. [DOI] [PMID]
25. Yin Y, Wang T, Zhang P, Li C, Yang W, Lin Y, et al. A Novel Model Predicts Postoperative Pathology of Colorectal High-Grade Intraepithelial Neoplasia. J Surg Res 2019; 240:104-8. [DOI] [PMID]
26. Wang CB, Shahjehan F, Merchea A, Li Z, Bekaii-Saab TS, Grothey A, et al. Impact of Tumor Location and Variables Associated with Overall Survival in Patients with Colorectal Cancer: A Mayo Clinic Colon and Rectal Cancer Registry Study. Front Oncol 2019; 9:76. [DOI] [PMID] [PMCID]
27. Fields AC, Lu P, Vierra BM, Hu F, Irani J, Bleday R, et al. Survival in Patients with High-Grade Colorectal Neuroendocrine Carcinomas: The Role of Surgery and Chemotherapy. Ann Surg Oncol 2019; 26(4):1127-33. [DOI] [PMID] [PMCID]
28. Kim S, Lee SC, Skinner CS, Brown CJ, Balentine CJ. A Surgeon’s Guide to Treating Older Patients with Colorectal Cancer. Curr Colorectal Cancer Rep 2019; 15(1):1-7. [DOI] [PMID] [PMCID]
29. Vallance AE, Young AL, Kuryba A, Braun M, Hill J, Jayne DG, et al. The impact of advancing age on incidence of hepatectomy and post-operative outcomes in patients with colorectal cancer liver metastases: a population-based cohort study. HPB (Oxford) 2019; 21(2):167-74. [DOI] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution 4.0 International License. |








