Volume 11, Issue 4 (Autumn 2022)
J Occup Health Epidemiol 2022, 11(4): 265-274 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khajehlandi M, Bolboli L, Bolboli S. The Role of Aerobic Exercise in the Gene Expression of Metallo Proteinase-2 and Angiostatin in Diabetic Rats' Heart Tissue. J Occup Health Epidemiol 2022; 11 (4) :265-274
URL: http://johe.rums.ac.ir/article-1-606-en.html
URL: http://johe.rums.ac.ir/article-1-606-en.html
Related article in
Google Scholar
Google Scholar
Similar articles
1- Ph.D, Dept. of Exercise Physiology, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran. , md.khajehlandi@uma.ac.ir
2- Associate Prof., Dept. of Exercise Physiology, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran
3- Medical Student, Faculty of Medicine, Balikesir University, Turkey.
2- Associate Prof., Dept. of Exercise Physiology, Faculty of Educational Sciences and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran
3- Medical Student, Faculty of Medicine, Balikesir University, Turkey.
Article history


Received: 2022/06/16
Accepted: 2022/12/1
ePublished: 2022/12/26
Accepted: 2022/12/1
ePublished: 2022/12/26
Subject:
Epidemiology
Full-Text [PDF 602 kb]
(663 Downloads)
| Abstract (HTML) (2059 Views)
Table 1. Training protocol
Table 2. The primers’ sequence for the quantitative RT-PCR
.jpg)
Fig.1. Blood glucose levels in different groups
Disparate characters (a, b, and c) represent a significant difference among the groups based on the one-way ANOVA test (P < 0.001). Accordingly, a represents a significant decrease in the DE group compared to the SD group; b represents a significant increase in the SD group compared to the HC group; and c represents a significant decrease in the HC group compared to the DE and SD groups. HC stands for the healthy control group, SD is the sedentary diabetic group, and DE is the diabetic exercise group.
.jpg)
Fig. 2. Heart weight in different groups
Disparate characters (a and b) represent a significant change among the groups based on the one-way ANOVA test (P < 0/001). Accordingly, a represents a significant increase in the DE and HC groups compared to the SD group; and b represents a significant decrease in the SD group compared to the HC and DE groups. HC is the healthy control group, SD stands for the sedentary diabetic group, and DT represents the diabetic exercise group.
.jpg)
Fig. 3. Serum cortisol levels in different groups
Disparate characters (a and b) represent a significant change among groups based on the one-way ANOVA test (P < 0/001); accordingly, a represents a significant decrease in the DE and HC groups compared to the SD group; and b represents a significant increase in the SD group compared to the HC and DE groups. HC is the healthy control group, SD represents the sedentary diabetic group, and DE is the diabetic training group.
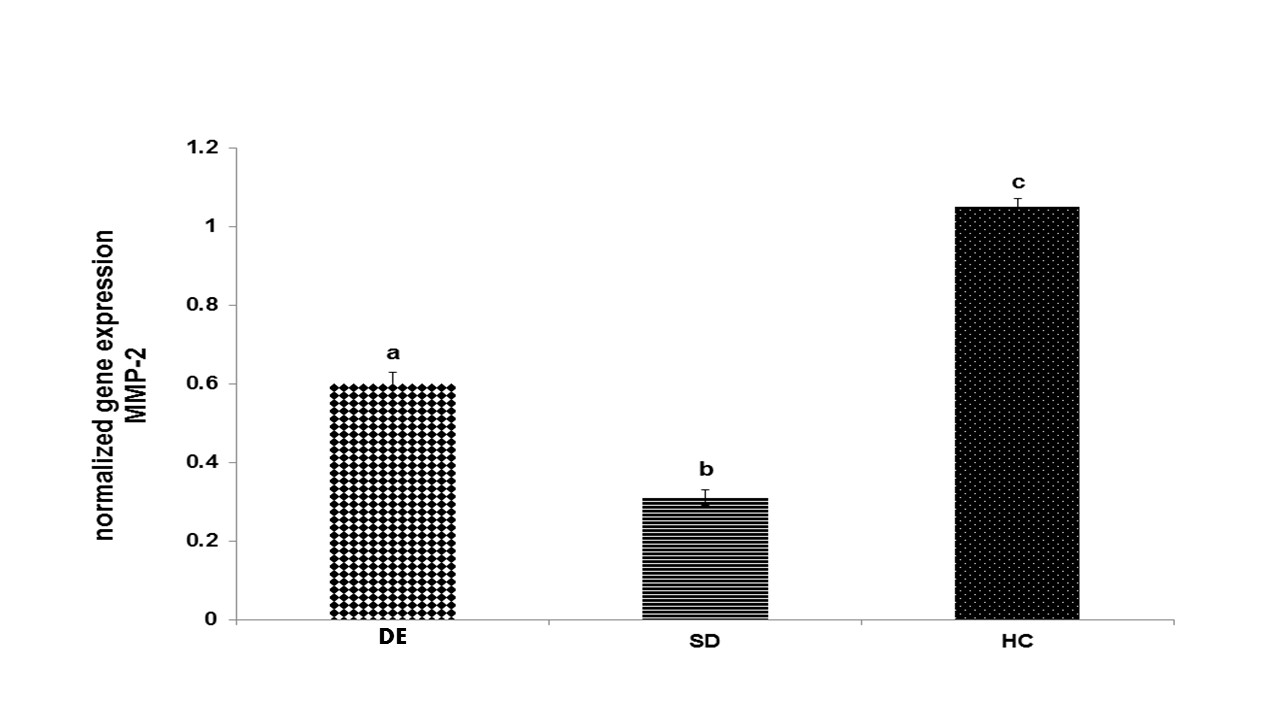
Fig. 4. Gene expression of MMP-2 in different groups
Disparate characters (a, b, and c) represent a significant change among the groups based on the one-way ANOVA test (P < 0.001); accordingly, a represents a significant increase in the DE group compared to the SD group; b represents a significant decrease in the SD group compared to the HC group, and c shows a significant increase in the HC group compared to the DE and SD groups. HC is the healthy control group, SD represents the sedentary diabetic group, and DE is the diabetic training group.
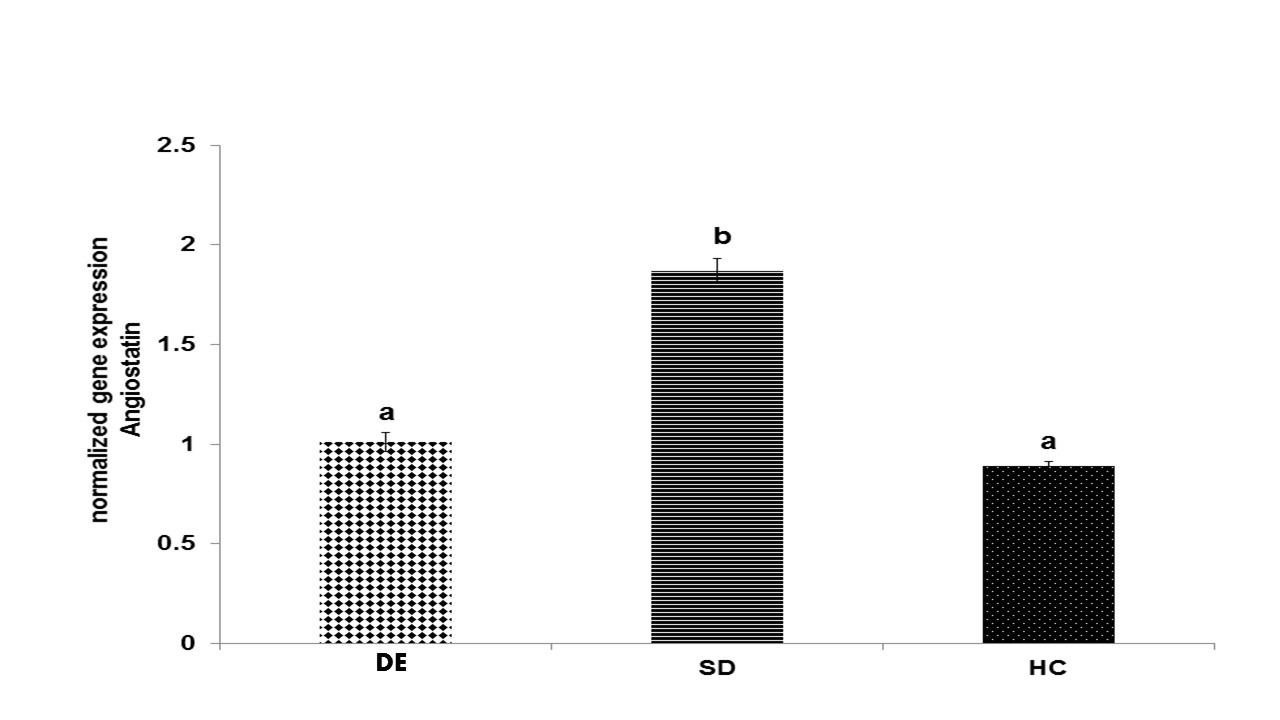
Fig. 5. Gene expression of angiostatin in different groups
Disparate characters (a and b) represent a significant change among the groups based on the one-way ANOVA test (P < 0.001); accordingly, a represents a significant decrease in the DE and HC groups compared to the SD group; b represents a significant increase in the SD group compared to the HC and DE groups. HC represents the healthy control group, SD is the sedentary diabetic group, and DE is the diabetic exercise group.
Full-Text: (285 Views)
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by perpetual hyperglycemia caused by the lack of insulin or insulin resistance [1]. In fact, it harms vessels’ function and structure, thereby leading to cardiovascular diseases (CVDs), with about 70% of diabetics suffering from CVDs [2, 3]. CVDs are among the major causes of death in the world, and unfortunately the number of CVD patients is increasing nowadays [4]. The mortality rate of CVDs in diabetics is 2-4 times higher than that in people without diabetes [5]. Collateral vessel formation and abnormal angiogenesis are the most prevalent cardiovascular symptoms in diabetics [6]. Diabetes conditions have paradoxical effects on vascular beds; accordingly, they decrease angiogenesis in coronary heart disease and peripheral nervous disorders. However, diabetes conditions increase angiogenesis in other organs, such as eyes [7].
Angiogenesis is a complex multi-step process that creates new capillaries controlled by both angiogenic and angiostatic components [3, 8, 9]. Besides, it is stimulated by some regulators, such as matrix metalloproteinases (MMPs), during vascular development [10]. A zinc-dependent protease, i.e. matrix metalloproteinase-2 (MMP-2) secreted by cardio myocytes, cardiac fibroblasts, and endocardial cells, is able to break down extracellular matrix proteins in the cardiovascular system [11], thereby spreading endothelial cells [12]. Research showed that the expression or activity of MMP-2 decreased in diabetic rats’ cardiac tissue [11, 13].
On the other hand, there is enough evidence indicating that a potent anti-angiogenic factor and angiostatin limit the spread and growth of new capillaries as well as endothelial and mesenchymal stem cells [14, 15]. Angiostatin specifically induces apoptosis in vascular cells by inhibiting their proliferation [15]. The binding of this potent antiangiogenic factor to the plasma membrane-localized ATP synthase suppresses ATP metabolism in vascular endothelial cells, with this downregulating endothelial cell proliferation [5]. The effects of angiostatin in patients with diabetic nephropathy and retinopathy have been investigated by some studies [16, 17]. Based on past research, the activity and gene expression of angiostatin increased in diabetics [17, 18].
Endothelial cell migration and proliferation are also influenced by a variety of other variables, such as growth hormone (GH), insulin-like growth factor (IGF-1), and other cytokines, including interleukins, as well as cortisol [19]. Cortisol has been indicated to be associated with impaired β-cell function and insulin resistance, which are known as the components of the molecular pathogenesis of DM [20]. Besides, cortisol may contribute to the progression of cardiac function and structure damage [21], although its relationship with angiogenesis has not been clearly investigated in diabetics.
Sedentary lifestyle as well as overweight and obesity, being major risk factors, are associated with DM [3]. Exercise, as a major safe factor in lifestyle, appears to be an effective, low-cost, and safe strategy for the treatment of diabetes [22]. Several studies report that heart tissue is affected by regular exercise [3, 23]. Positive effects of aerobic exercise on heart failure and coronary artery disease in DM are attributed to the improvement in insulin sensitivity, glycemic status, and plasma lipids [24, 25]. In fact, aerobic exercise and DM are associated with changes in gene expression patterns. To date, the effects of different types of exercise on cell signaling events, including changes in gene activity and protein modifications in the heart tissue with or without diabetic cardiomyopathy, have not been known completely. In addition, research results have been inconsistent with different outcomes [7, 26, 27].
There are few studies consistently reporting significant improvements in the cardiac function and structure in diabetics [28, 29]; however, animal studies indicate beneficial effects and structural alterations in the heart tissue of healthy people and diabetics [3, 7]. According to the study by Ardakanizade et al (2018), after long-term anaerobic exercise, the gene expression levels of MMP-2 increased in the left ventricle of Wistar rats [7]. However, in a study by Ghorbanalipour et al (2020), angiostatin decreased after 10 weeks of anaerobic exercise, inducing gene expression in diabetic rats’ cardiac tissue [17]. In contrast, in a study by Kadoglou et al (2010), the effects of exercise on MMP levels in type 2 diabetics were examined, with no significant changes observed in the serum levels of MMP-2 after 12 weeks of exercise [27]. In this regard, there are few studies that have examined the effects of moderate-intensity anaerobic exercise on MMP-2 and angiostatin gene expression. Therefore, it seems that more studies are required in this field. In fact, therapeutic effects of anaerobic exercise interventions have been proved on the cellular signaling process in diabetic heart tissue. In addition, identification of exercise-induced molecular mechanisms is an important step towards better evaluation of the effectiveness of different training interventions. Thus, understanding molecular mechanisms induced by anaerobic exercise may help identify novel therapeutic targets. Against this backdrop, the present study was conducted to determine if moderate-intensity anaerobic exercise could improve MMP-2 and angiostatin gene expression in diabetic rats' heart tissue.
Materials and Methods
This experimental study was approved by the Ethics Committee on the use of animals at Ardabil University of Medical Sciences (IR.ARUMS.REC.1398.251).
Animals and grouping: A total of 30 adult male Wistar rats aging 10 weeks and weighing 245 ± 9/4 gr were used in this study. The rats were purchased from the Physiology Research Center of Lorestan University of Medical Sciences, Iran. In fact, all procedures were followed with the approval of the Ethics Committee of Ardabil University of Medical Sciences, under ethics code IR.ARUMS.REC.1398.251. The animals were placed in separate cages with free access to water and food (three animals in each cage). Next, they were familiarized with the treadmill under laboratory conditions for 2 weeks [30]. During the familiarization process, all animals performed treadmill walking at speed 10 m/min for 10-15 min, 5 days a week. After the familiarization stage, the rats were checked for their weight and were randomly divided into 3 groups. Accordingly, the groups included diabetic exercise (DE), sedentary diabetic (SD), and healthy controls (HC) groups. The two last groups were kept in standard cages without running wheels during 6 weeks.
Materials and drugs: Streptozotocin (STZ) and citrate solution were purchased from Sigma (USA). In addition, Xylazine and Ketamine were provided from Alfasan Pharmaceutical Co. (Nederland). Diabetes was induced by intraperitoneal injection of 50 mg/kg of STZ solution prepared in the fresh citrate buffer 0.5 M at pH 4.5. Besides, the same volume of the citrate solution was administered to non-diabetic animals.
Diabetes animal model: The rats were kept in Plexiglas cages under controlled environmental conditions of humidity (35 ± 5%) and at a stable temperature (23 ± 5 °C) in a 12-hour light-dark cycle. Diabetes was induced following overnight fasting. Next, 72 hours after the injection, a small blood drop was placed on the glucometer tape and read by a glucometer (Roche Diagnostics K.K., Tokyo, Japan). The rats would be considered diabetic if their blood glucose levels exceeded 250 mg/dL [31]. To prove that the non-diabetic rats’ blood glucose was normoglycemic, its level was measured at the beginning. In addition, the blood glucose levels of the three groups were controlled throughout the research.
Endurance training protocol: Given the risk of cardiovascular events, we chose moderate-intensity aerobic exercise to avoid a large increase in the rats’ blood pressure and minimize the risk of cardiovascular overload. Animals in the aerobic exercise group were acclimated to treadmill running and ran in wholly separated lanes. After the 1st week of adaptation, according to our moderate-intensity aerobic exercise protocol, all rats in the DE group took aerobic exercise on a treadmill for 5 weeks (5 days a week). Before and after the exercise, 2 to 3 min of warmup and cool-down periods were considered, respectively. In all stages, the treadmill slope was zero; however, the speed and duration of the treadmill gradually increased from 10 m/min for 10 min in the 1st week to 10 m/min for 20 min in the 2nd week, 15 m/min for 20 min in the 3rd week, 15 m/min for 30 min in the 4th week, and 18-17 m/min for 30 min in the 5th week. To turn adaptations into a steady state, all exercise variables were kept constant during the final week (6th week) (Table 1). Aerobic exercise was taken at 08:00-12:00 am. In addition, 20 sedentary rats (SD and HC groups) were kept in 7 cages in a separate room during the experiment.
Diabetes mellitus (DM) is a metabolic disorder characterized by perpetual hyperglycemia caused by the lack of insulin or insulin resistance [1]. In fact, it harms vessels’ function and structure, thereby leading to cardiovascular diseases (CVDs), with about 70% of diabetics suffering from CVDs [2, 3]. CVDs are among the major causes of death in the world, and unfortunately the number of CVD patients is increasing nowadays [4]. The mortality rate of CVDs in diabetics is 2-4 times higher than that in people without diabetes [5]. Collateral vessel formation and abnormal angiogenesis are the most prevalent cardiovascular symptoms in diabetics [6]. Diabetes conditions have paradoxical effects on vascular beds; accordingly, they decrease angiogenesis in coronary heart disease and peripheral nervous disorders. However, diabetes conditions increase angiogenesis in other organs, such as eyes [7].
Angiogenesis is a complex multi-step process that creates new capillaries controlled by both angiogenic and angiostatic components [3, 8, 9]. Besides, it is stimulated by some regulators, such as matrix metalloproteinases (MMPs), during vascular development [10]. A zinc-dependent protease, i.e. matrix metalloproteinase-2 (MMP-2) secreted by cardio myocytes, cardiac fibroblasts, and endocardial cells, is able to break down extracellular matrix proteins in the cardiovascular system [11], thereby spreading endothelial cells [12]. Research showed that the expression or activity of MMP-2 decreased in diabetic rats’ cardiac tissue [11, 13].
On the other hand, there is enough evidence indicating that a potent anti-angiogenic factor and angiostatin limit the spread and growth of new capillaries as well as endothelial and mesenchymal stem cells [14, 15]. Angiostatin specifically induces apoptosis in vascular cells by inhibiting their proliferation [15]. The binding of this potent antiangiogenic factor to the plasma membrane-localized ATP synthase suppresses ATP metabolism in vascular endothelial cells, with this downregulating endothelial cell proliferation [5]. The effects of angiostatin in patients with diabetic nephropathy and retinopathy have been investigated by some studies [16, 17]. Based on past research, the activity and gene expression of angiostatin increased in diabetics [17, 18].
Endothelial cell migration and proliferation are also influenced by a variety of other variables, such as growth hormone (GH), insulin-like growth factor (IGF-1), and other cytokines, including interleukins, as well as cortisol [19]. Cortisol has been indicated to be associated with impaired β-cell function and insulin resistance, which are known as the components of the molecular pathogenesis of DM [20]. Besides, cortisol may contribute to the progression of cardiac function and structure damage [21], although its relationship with angiogenesis has not been clearly investigated in diabetics.
Sedentary lifestyle as well as overweight and obesity, being major risk factors, are associated with DM [3]. Exercise, as a major safe factor in lifestyle, appears to be an effective, low-cost, and safe strategy for the treatment of diabetes [22]. Several studies report that heart tissue is affected by regular exercise [3, 23]. Positive effects of aerobic exercise on heart failure and coronary artery disease in DM are attributed to the improvement in insulin sensitivity, glycemic status, and plasma lipids [24, 25]. In fact, aerobic exercise and DM are associated with changes in gene expression patterns. To date, the effects of different types of exercise on cell signaling events, including changes in gene activity and protein modifications in the heart tissue with or without diabetic cardiomyopathy, have not been known completely. In addition, research results have been inconsistent with different outcomes [7, 26, 27].
There are few studies consistently reporting significant improvements in the cardiac function and structure in diabetics [28, 29]; however, animal studies indicate beneficial effects and structural alterations in the heart tissue of healthy people and diabetics [3, 7]. According to the study by Ardakanizade et al (2018), after long-term anaerobic exercise, the gene expression levels of MMP-2 increased in the left ventricle of Wistar rats [7]. However, in a study by Ghorbanalipour et al (2020), angiostatin decreased after 10 weeks of anaerobic exercise, inducing gene expression in diabetic rats’ cardiac tissue [17]. In contrast, in a study by Kadoglou et al (2010), the effects of exercise on MMP levels in type 2 diabetics were examined, with no significant changes observed in the serum levels of MMP-2 after 12 weeks of exercise [27]. In this regard, there are few studies that have examined the effects of moderate-intensity anaerobic exercise on MMP-2 and angiostatin gene expression. Therefore, it seems that more studies are required in this field. In fact, therapeutic effects of anaerobic exercise interventions have been proved on the cellular signaling process in diabetic heart tissue. In addition, identification of exercise-induced molecular mechanisms is an important step towards better evaluation of the effectiveness of different training interventions. Thus, understanding molecular mechanisms induced by anaerobic exercise may help identify novel therapeutic targets. Against this backdrop, the present study was conducted to determine if moderate-intensity anaerobic exercise could improve MMP-2 and angiostatin gene expression in diabetic rats' heart tissue.
Materials and Methods
This experimental study was approved by the Ethics Committee on the use of animals at Ardabil University of Medical Sciences (IR.ARUMS.REC.1398.251).
Animals and grouping: A total of 30 adult male Wistar rats aging 10 weeks and weighing 245 ± 9/4 gr were used in this study. The rats were purchased from the Physiology Research Center of Lorestan University of Medical Sciences, Iran. In fact, all procedures were followed with the approval of the Ethics Committee of Ardabil University of Medical Sciences, under ethics code IR.ARUMS.REC.1398.251. The animals were placed in separate cages with free access to water and food (three animals in each cage). Next, they were familiarized with the treadmill under laboratory conditions for 2 weeks [30]. During the familiarization process, all animals performed treadmill walking at speed 10 m/min for 10-15 min, 5 days a week. After the familiarization stage, the rats were checked for their weight and were randomly divided into 3 groups. Accordingly, the groups included diabetic exercise (DE), sedentary diabetic (SD), and healthy controls (HC) groups. The two last groups were kept in standard cages without running wheels during 6 weeks.
Materials and drugs: Streptozotocin (STZ) and citrate solution were purchased from Sigma (USA). In addition, Xylazine and Ketamine were provided from Alfasan Pharmaceutical Co. (Nederland). Diabetes was induced by intraperitoneal injection of 50 mg/kg of STZ solution prepared in the fresh citrate buffer 0.5 M at pH 4.5. Besides, the same volume of the citrate solution was administered to non-diabetic animals.
Diabetes animal model: The rats were kept in Plexiglas cages under controlled environmental conditions of humidity (35 ± 5%) and at a stable temperature (23 ± 5 °C) in a 12-hour light-dark cycle. Diabetes was induced following overnight fasting. Next, 72 hours after the injection, a small blood drop was placed on the glucometer tape and read by a glucometer (Roche Diagnostics K.K., Tokyo, Japan). The rats would be considered diabetic if their blood glucose levels exceeded 250 mg/dL [31]. To prove that the non-diabetic rats’ blood glucose was normoglycemic, its level was measured at the beginning. In addition, the blood glucose levels of the three groups were controlled throughout the research.
Endurance training protocol: Given the risk of cardiovascular events, we chose moderate-intensity aerobic exercise to avoid a large increase in the rats’ blood pressure and minimize the risk of cardiovascular overload. Animals in the aerobic exercise group were acclimated to treadmill running and ran in wholly separated lanes. After the 1st week of adaptation, according to our moderate-intensity aerobic exercise protocol, all rats in the DE group took aerobic exercise on a treadmill for 5 weeks (5 days a week). Before and after the exercise, 2 to 3 min of warmup and cool-down periods were considered, respectively. In all stages, the treadmill slope was zero; however, the speed and duration of the treadmill gradually increased from 10 m/min for 10 min in the 1st week to 10 m/min for 20 min in the 2nd week, 15 m/min for 20 min in the 3rd week, 15 m/min for 30 min in the 4th week, and 18-17 m/min for 30 min in the 5th week. To turn adaptations into a steady state, all exercise variables were kept constant during the final week (6th week) (Table 1). Aerobic exercise was taken at 08:00-12:00 am. In addition, 20 sedentary rats (SD and HC groups) were kept in 7 cages in a separate room during the experiment.
Table 1. Training protocol
| Weeks (acclimatization) | 1 |
2 | 3 | 4 | 5 | 6 |
| Speed (m/min) | 10 | 20 | 20 | 30 | 30 | 30 |
| Time (min) | 10 | 10 | 15 | 15 | 17-18 | 17-18 |
| Slope | 0 | 0 | 0 | 0 | 0 | 0 |
Biochemical assays: To measure cortisol concentrations, all animals were sacrificed 24 h after the last ET session by intraperitoneal injection of ketamine (75 mg/kg) and xylazine (5 mg/kg) after 12-h fasting. Next, blood samples were taken from the animals' hearts and centrifuged for 15 m at 3000 rpm to obtain serums. The serums were stored at −20 °C before going through analysis. Cortisol concentrations were determined using an ELISA kit (Diagnostic, Canada) in a multiple ELISA reader (BioTek, Winooski, VT, USA), according to the manufacturer's instructions.
Heart tissue extraction and RT-PCR: Each rat’s heart tissue was removed, submerged in liquid nitrogen, and kept at -70 °C. In addition, RNA extraction was performed by RNXTM reagent according to the manufacturer’s procedure (SinaClon Bioscience, Tehran, Iran). The supernatant was removed after homogenizing 100 mg of myocardium in one mL of Isol-RNA Lysis Reagent. Next, it was transferred to a micro tube, with the homogeneous product centrifuged for 10 min at 12000 g (4 °C). At the next stage, chloroform was added to the supernatant at 200 μL and vigorously stirred for 15 s. Next, the microtubes were re-centrifuged for 15 min at 12000 g (4 °C). In addition, 600 μL of isopropyl alcohol was added and centrifuged at 12000 g, with the RNA extracted. The purity and concentration of the RNA were calculated by controlling the ratio of OD260/280, and values of acceptable purity were defined between 1.8 and 2. The extracted RNA was reversed to complementary DNA (c-DNA). Additionally, the cDNA synthesis kit was used for cDNA synthesis, according to the manufacturer’s instructions. The real-time PCR was performed in the Roche LightCycler detection system (Basel, Switzerland). For this purpose, initial denaturation of 5 min at 95 °C, 45 cycles of denaturation for 15 s at 95 °C, annealing for 30 s at 60 °C, an extension for 20 s at 72 °C, and a melt curve analysis (50–99 °C) were performed. Besides, to measure relative gene expression, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene to control the product’s specific proliferation. The results were evaluated using the -2ΔΔCt comparative method as well as LightCycler SW1.1 software and were reported using the general -2ΔΔCt formula. Table 2 shows the sequence of the primers. To measure glucose levels, a small drop of blood was placed on the glucometer tape by creating a small lesion on the venous tail vein, with blood glucose levels read by a glucometer (Roche Diagnostics K.K., Tokyo, Japan). The rats’ hearts were weighed by a GR202 scale made in Japan.
Heart tissue extraction and RT-PCR: Each rat’s heart tissue was removed, submerged in liquid nitrogen, and kept at -70 °C. In addition, RNA extraction was performed by RNXTM reagent according to the manufacturer’s procedure (SinaClon Bioscience, Tehran, Iran). The supernatant was removed after homogenizing 100 mg of myocardium in one mL of Isol-RNA Lysis Reagent. Next, it was transferred to a micro tube, with the homogeneous product centrifuged for 10 min at 12000 g (4 °C). At the next stage, chloroform was added to the supernatant at 200 μL and vigorously stirred for 15 s. Next, the microtubes were re-centrifuged for 15 min at 12000 g (4 °C). In addition, 600 μL of isopropyl alcohol was added and centrifuged at 12000 g, with the RNA extracted. The purity and concentration of the RNA were calculated by controlling the ratio of OD260/280, and values of acceptable purity were defined between 1.8 and 2. The extracted RNA was reversed to complementary DNA (c-DNA). Additionally, the cDNA synthesis kit was used for cDNA synthesis, according to the manufacturer’s instructions. The real-time PCR was performed in the Roche LightCycler detection system (Basel, Switzerland). For this purpose, initial denaturation of 5 min at 95 °C, 45 cycles of denaturation for 15 s at 95 °C, annealing for 30 s at 60 °C, an extension for 20 s at 72 °C, and a melt curve analysis (50–99 °C) were performed. Besides, to measure relative gene expression, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene to control the product’s specific proliferation. The results were evaluated using the -2ΔΔCt comparative method as well as LightCycler SW1.1 software and were reported using the general -2ΔΔCt formula. Table 2 shows the sequence of the primers. To measure glucose levels, a small drop of blood was placed on the glucometer tape by creating a small lesion on the venous tail vein, with blood glucose levels read by a glucometer (Roche Diagnostics K.K., Tokyo, Japan). The rats’ hearts were weighed by a GR202 scale made in Japan.
Table 2. The primers’ sequence for the quantitative RT-PCR
| Genes | Forward | Reverse |
| GAPDH | AGTTCAACGGCACAGTCAAG | TACTCAGCACCAGCATCACC |
| MMP-2 | CTACACTGGGACCTGTCACT | CCGCCAAATAAACCGATCCT |
| Angiostatin | ACCTGCTAGACCACCTGGAG | CCTTGGCTGTTATCTTCGGTACCGG |
Normal distribution of the data and homogeneity of variances were evaluated using the Shapiro-Wilk and Levene tests, respectively (P ≥ 0.05). Next, a one-way ANOVA and the Tukey’s post-hoc test were used to compare changes in different groups. All statistical analyses were performed at the significance level of P < 0.05 using SPSS software V.25.0.
Results
Rats’ blood glucose levels, heart weight, and
cortisol concentrations: Blood glucose levels in the SD group increased significantly during the experimental period (P ˂ 0/001). However, there was no significant change in blood glucose levels in the HC group (P ≥ 0/05). The results of statistical analysis using a one-way ANOVA showed that at the end of 6 weeks of moderate-intensity aerobic exercise, blood glucose concentrations were significantly lower in the DE group than in the SD group (P ˂ 0/001) (Fig. 1). However, there was a significant decrease in the DE and HC groups (P ˂ 0/001) as well.
Results
Rats’ blood glucose levels, heart weight, and
cortisol concentrations: Blood glucose levels in the SD group increased significantly during the experimental period (P ˂ 0/001). However, there was no significant change in blood glucose levels in the HC group (P ≥ 0/05). The results of statistical analysis using a one-way ANOVA showed that at the end of 6 weeks of moderate-intensity aerobic exercise, blood glucose concentrations were significantly lower in the DE group than in the SD group (P ˂ 0/001) (Fig. 1). However, there was a significant decrease in the DE and HC groups (P ˂ 0/001) as well.
.jpg)
Fig.1. Blood glucose levels in different groups
Disparate characters (a, b, and c) represent a significant difference among the groups based on the one-way ANOVA test (P < 0.001). Accordingly, a represents a significant decrease in the DE group compared to the SD group; b represents a significant increase in the SD group compared to the HC group; and c represents a significant decrease in the HC group compared to the DE and SD groups. HC stands for the healthy control group, SD is the sedentary diabetic group, and DE is the diabetic exercise group.
There was a significant difference in heart weight among the three groups at the end of the 6 weeks (P ˂ 0/001). Accordingly, heart weight was lower in the SD group than in the HC and DE groups (P ˂ 0/001). Nevertheless, there was no significant difference between HC and DE groups (P ≥ 0/05) (Fig. 2).
.jpg)
Fig. 2. Heart weight in different groups
Disparate characters (a and b) represent a significant change among the groups based on the one-way ANOVA test (P < 0/001). Accordingly, a represents a significant increase in the DE and HC groups compared to the SD group; and b represents a significant decrease in the SD group compared to the HC and DE groups. HC is the healthy control group, SD stands for the sedentary diabetic group, and DT represents the diabetic exercise group.
In fact, there was a significant difference in the serum levels of cortisol among the three groups at the end of 6 weeks (P ˂ 0/001). Accordingly, serum levels of cortisol were higher in the SD group than in the HC and DE groups (P ˂ 0/001). However, there was no significant difference between the HC and DE groups (P ≥ 0/05) (Fig. 3).
.jpg)
Fig. 3. Serum cortisol levels in different groups
Disparate characters (a and b) represent a significant change among groups based on the one-way ANOVA test (P < 0/001); accordingly, a represents a significant decrease in the DE and HC groups compared to the SD group; and b represents a significant increase in the SD group compared to the HC and DE groups. HC is the healthy control group, SD represents the sedentary diabetic group, and DE is the diabetic training group.
Gene expression of MMP-2 and angiostatin: Figs. 4 and 5 show the results of the one-way ANOVA and the Tukey’s post-hoc test for the gene comparison of MMP-2 after 6 weeks of moderate-intensity aerobic exercise. Accordingly, gene expression of MMP-2 had a significant decrease in the SD group compared to the HC group (P < 0/001). Besides, there was a significant increase in the DE group compared to the SD group (P < 0/001). However, there was a significant difference between the DE and HC groups (P < 0/001) (Fig. 4).

Fig. 4. Gene expression of MMP-2 in different groups
Disparate characters (a, b, and c) represent a significant change among the groups based on the one-way ANOVA test (P < 0.001); accordingly, a represents a significant increase in the DE group compared to the SD group; b represents a significant decrease in the SD group compared to the HC group, and c shows a significant increase in the HC group compared to the DE and SD groups. HC is the healthy control group, SD represents the sedentary diabetic group, and DE is the diabetic training group.
In fact, gene expression of angiostatin had a significant increase in the SD group compared to the HC group (P < 0/001). However, it had a significant decrease in the DE group compared to the SD group (P < 0/001) (Fig. 5). Nevertheless, there was no significant difference between the HC and DE groups (P ≥ 0/05).

Fig. 5. Gene expression of angiostatin in different groups
Disparate characters (a and b) represent a significant change among the groups based on the one-way ANOVA test (P < 0.001); accordingly, a represents a significant decrease in the DE and HC groups compared to the SD group; b represents a significant increase in the SD group compared to the HC and DE groups. HC represents the healthy control group, SD is the sedentary diabetic group, and DE is the diabetic exercise group.
Discussion
Diabetics are at an increased risk of developing CVDs. Nowadays, glycemic variability is considered a noticeable causative factor for the development of DM [3]. Animal and in vitro studies show that exercise improves the function and structure of the heart by better glucose control, thereby promoting cardiovascular health [32]. In fact, regular exercise is an effective non-pharmacological approach for the management of disorders among diabetics. The main findings of the present study were a reduction in blood glucose and cortisol levels as well as an improvement in the gene expression of MMP-2 and angiostatin. Although fasting blood glucose levels were significantly higher in diabetic groups, moderate-intensity aerobic exercise controlled serum levels of glucose during the experiment as expected, without restoring normoglycemia. A reduction in the blood glucose level from 364 to 325 mg/dL may reduce the risk of cardiovascular events. To date, regulatory blood glucose mechanisms of aerobic exercise in diabetics have remained unclear. Probable changes in the target proteins can affect glucose regulation in the long term, which is associated with insulin sensitivity. According to research, a reduction in the blood glucose level and an improvement in insulin sensitivity triggered anti-inflammatory processes and affected oxidative capacity as well as capillarization [33, 34]. In the current investigation, heart weight in two groups of the diabetic rats decreased significantly. In fact, this effect was more significant in the SD group than in the DE group, indicating that aerobic exercise somehow controls heart tissue atrophy.
A variety of factors are required to initiate heart angiogenesis in response to exercise, with one of which being serum cortisol levels. Based on the results of this study, serum cortisol levels decreased after 6 weeks of aerobic exercise. This result was consistent with some previous findings [35, 36], but it was inconsistent with some other findings [37, 38]. This inconsistency could have been due to the different subjects, obesity, inactivity, healthiness, and sickness. Accordingly, these differences may be associated with different exercise variables, including the intensity, volume, repetition, and type of exercise. The decline in glucose levels could be the major possible mechanism that decreases serum cortisol concentrations. This is due to the fact that the activity of the central sympathetic nervous system and the adrenal-hypophyseal-hypothalamic axis contribute to endocrine disorders, such as blood glucose increase [39]. Fortunately, in the present study, blood glucose levels decreased during and after the 6 weeks of the aerobic exercise, which affected adrenocorticotropic hormone (ACTH) [40] from the hypothalamic-pituitary axis, which controls cortisol secretion.
The main result of this study was that after the injection of STZ, the gene expression of MMP-2 and angiostatin remarkably decreased and increased, respectively, in the diabetic cardiac group. In addition, the 6 weeks of systemic aerobic exercise tended to decrease that. This result indicates the supportive effect of moderate-intensity aerobic exercise on diabetic heart angiogenesis through balancing gene expression of angiogenesis-related factors, or probably predominance of pro-angiogenic factors relative to anti-angiogenic factors as well as fibrosis due to diabetes in the diabetic cardiac group [3]. As observed in our study, after 6 weeks of aerobic exercise, gene expression of MMP-2 increased, having been in line with some other studies [41, 42]. Habibian et al (2016) reported that after 8 weeks of swimming, MMP-2 cardiac activity increased [41]. In the same vein, Kwak et al (2011) reported that after 12 weeks of aerobic exercise, MMP-2 gen activity increased in the left ventricular [42]. Elevated glucose levels can facilitate growth factor β1 (TGFβ1) activation by increasing the synthesis of anti-angiogenic agents, such as thrombospondin-1 (TSP1). In addition, profibrotic factor TGFβ1 can accelerate accumulation of the extracellular matrix by mediating inflammatory responses [43]. Therefore, increasing gene expression of MMP-2 in cardiac tissue and decreasing blood glucose levels in the rats that took exercise in the present study would confirm the cardiac supportive role of aerobic exercise under diabetes conditions. It has been suggested that elevated levels of glucose, angiotensin II, and oxidative stress can cause structural changes in diabetics’ heart by increasing MMP-2 [44, 45] and decreasing angiostatin. However, in the present study, the two mentioned factors (angiotensin II and oxidative stress) were not investigated, which could be one of the limitations of the present research. Fortunately, a decrease in angiotensin II and oxidative stress has been reported following aerobic exercise [46, 47]. As few studies have examined MMP-2 signaling and its activity in normal or diabetic heart tissue, discussion in this regard is limited.
In this study, angiostatin regulation increased in the diabetic group. Therefore, our study confirms previous studies which reported a significant change in the gene expression of angiostatin in the diabetic heart of rats compared to the healthy heart [48]. In this regard, it is indicated that angiostatin inhibited angiogenesis of pathological conditions as well, yet it had no effect on general physiological angiogenesis [49]. It is not well known how DM increases angiostatin. However, it has been indicated that the activity of phosphoglycerate kinase (PKG) increased under diabetes conditions following an increase in angiotensin expression. The association between increased angiostatin and PGK efficacy could be due to the fact that plasmin revival is the first step in angiostatin formation. In a study, the enzyme PGK facilitated plasmin reduction, with the increase in PGK activity stated as the reason for the increase in angiotensin expression [18]. Although the expression of the angiostatin gene increased in diabetics [18, 48], fortunately 6 weeks of aerobic exercise decreased its gene expression. There is not enough clinical research to have examined angiostatin responses to exercise; however, laboratory findings show that 10 weeks of exercise (at the rate of 17 m/min for 10-50 min) reduced levels of angiostatin and caspase-3 gene expression, thereby increasing the capillary density of arteries in mice with myocardial infarction [48]. The reason for angiostatin reduction in response to exercise is not clearly known. In fact, a reduction in MMPs probably reduced release of angiostatin from plasminogen after exercise, due to the relationship between MMP activity and nitric oxide production [50]. The result of a study showed that coronary collateralization was dependent on nitric oxide, which in part severely restricted the activity of MMPs and subsequent angiostatin production from plasminogen [50]. Therefore, changes in nitric oxide could be effective in angiostatin production, yet its amount was not measured in the present study; however, its activity increased after exercise in most studies [51, 52].
Conclusion
In summary, systemic moderate-intensity aerobic exercise in diabetic rats exerted beneficial effects on the diabetic heart disease, controlled blood glucose, increased MMP-2 gene expression, and decreased angiostatin gene expression. These benefits could help improve the angiogenesis process, form new capillaries, and increase blood flow to the affected areas in diabetics.
Acknowledgement
This study was funded by the current researchers.
Conflict of interest: None declared.
A variety of factors are required to initiate heart angiogenesis in response to exercise, with one of which being serum cortisol levels. Based on the results of this study, serum cortisol levels decreased after 6 weeks of aerobic exercise. This result was consistent with some previous findings [35, 36], but it was inconsistent with some other findings [37, 38]. This inconsistency could have been due to the different subjects, obesity, inactivity, healthiness, and sickness. Accordingly, these differences may be associated with different exercise variables, including the intensity, volume, repetition, and type of exercise. The decline in glucose levels could be the major possible mechanism that decreases serum cortisol concentrations. This is due to the fact that the activity of the central sympathetic nervous system and the adrenal-hypophyseal-hypothalamic axis contribute to endocrine disorders, such as blood glucose increase [39]. Fortunately, in the present study, blood glucose levels decreased during and after the 6 weeks of the aerobic exercise, which affected adrenocorticotropic hormone (ACTH) [40] from the hypothalamic-pituitary axis, which controls cortisol secretion.
The main result of this study was that after the injection of STZ, the gene expression of MMP-2 and angiostatin remarkably decreased and increased, respectively, in the diabetic cardiac group. In addition, the 6 weeks of systemic aerobic exercise tended to decrease that. This result indicates the supportive effect of moderate-intensity aerobic exercise on diabetic heart angiogenesis through balancing gene expression of angiogenesis-related factors, or probably predominance of pro-angiogenic factors relative to anti-angiogenic factors as well as fibrosis due to diabetes in the diabetic cardiac group [3]. As observed in our study, after 6 weeks of aerobic exercise, gene expression of MMP-2 increased, having been in line with some other studies [41, 42]. Habibian et al (2016) reported that after 8 weeks of swimming, MMP-2 cardiac activity increased [41]. In the same vein, Kwak et al (2011) reported that after 12 weeks of aerobic exercise, MMP-2 gen activity increased in the left ventricular [42]. Elevated glucose levels can facilitate growth factor β1 (TGFβ1) activation by increasing the synthesis of anti-angiogenic agents, such as thrombospondin-1 (TSP1). In addition, profibrotic factor TGFβ1 can accelerate accumulation of the extracellular matrix by mediating inflammatory responses [43]. Therefore, increasing gene expression of MMP-2 in cardiac tissue and decreasing blood glucose levels in the rats that took exercise in the present study would confirm the cardiac supportive role of aerobic exercise under diabetes conditions. It has been suggested that elevated levels of glucose, angiotensin II, and oxidative stress can cause structural changes in diabetics’ heart by increasing MMP-2 [44, 45] and decreasing angiostatin. However, in the present study, the two mentioned factors (angiotensin II and oxidative stress) were not investigated, which could be one of the limitations of the present research. Fortunately, a decrease in angiotensin II and oxidative stress has been reported following aerobic exercise [46, 47]. As few studies have examined MMP-2 signaling and its activity in normal or diabetic heart tissue, discussion in this regard is limited.
In this study, angiostatin regulation increased in the diabetic group. Therefore, our study confirms previous studies which reported a significant change in the gene expression of angiostatin in the diabetic heart of rats compared to the healthy heart [48]. In this regard, it is indicated that angiostatin inhibited angiogenesis of pathological conditions as well, yet it had no effect on general physiological angiogenesis [49]. It is not well known how DM increases angiostatin. However, it has been indicated that the activity of phosphoglycerate kinase (PKG) increased under diabetes conditions following an increase in angiotensin expression. The association between increased angiostatin and PGK efficacy could be due to the fact that plasmin revival is the first step in angiostatin formation. In a study, the enzyme PGK facilitated plasmin reduction, with the increase in PGK activity stated as the reason for the increase in angiotensin expression [18]. Although the expression of the angiostatin gene increased in diabetics [18, 48], fortunately 6 weeks of aerobic exercise decreased its gene expression. There is not enough clinical research to have examined angiostatin responses to exercise; however, laboratory findings show that 10 weeks of exercise (at the rate of 17 m/min for 10-50 min) reduced levels of angiostatin and caspase-3 gene expression, thereby increasing the capillary density of arteries in mice with myocardial infarction [48]. The reason for angiostatin reduction in response to exercise is not clearly known. In fact, a reduction in MMPs probably reduced release of angiostatin from plasminogen after exercise, due to the relationship between MMP activity and nitric oxide production [50]. The result of a study showed that coronary collateralization was dependent on nitric oxide, which in part severely restricted the activity of MMPs and subsequent angiostatin production from plasminogen [50]. Therefore, changes in nitric oxide could be effective in angiostatin production, yet its amount was not measured in the present study; however, its activity increased after exercise in most studies [51, 52].
Conclusion
In summary, systemic moderate-intensity aerobic exercise in diabetic rats exerted beneficial effects on the diabetic heart disease, controlled blood glucose, increased MMP-2 gene expression, and decreased angiostatin gene expression. These benefits could help improve the angiogenesis process, form new capillaries, and increase blood flow to the affected areas in diabetics.
Acknowledgement
This study was funded by the current researchers.
Conflict of interest: None declared.
References
1. Sharma AK, Thanikachalam PV, Rajput SK. Albiglutide: Is a better hope against diabetes mellitus? Biomed Pharmacother. 2016 ;77:120-8. [DOI] [PMID]
2. Bachmann KN, Wang TJ. Biomarkers of cardiovascular disease: contributions to risk prediction in individuals with diabetes. Diabetologia. 2018;61(5):987-95. [DOI] [PMID] [PMCID]
3. Khajehlandi M, Bolboli L, Siahkuhian M, Rami M, Tabandeh M, Khoramipour K, et al. Endurance Training Regulates Expression of Some Angiogenesis-Related Genes in Cardiac Tissue of Experimentally Induced Diabetic Rats. Biomolecules. 2021;11(4):498. [DOI] [PMID] [PMCID]
4. Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22. [DOI] [PMID] [PMCID]
5. Moser TL, Stack MS, Asplin I, Enghild JJ, Højrup P, Everitt L, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96(6):2811-6. [DOI] [PMID] [PMCID]
6. Hou R, Shen M, Wang R, Liu H, Gao C, Xu J, et al. Thioredoxin1 Inactivation Mediates the Impairment of Ischemia-Induced Angiogenesis and Further Injury in Diabetic Myocardium. J Vasc Res. 2020;57(2):76-85. [DOI] [PMID]
7. Ardakanizade M. The effects of mid- and long-term endurance exercise on heart angiogenesis and oxidative stress. Iran J Basic Med Sci. 2018;21(8):800-5. [DOI] [PMID] [PMCID]
9. Simunovic F, Finkenzeller G. Vascularization Strategies in Bone Tissue Engineering. Cells. 2021;10(7):1749. [DOI] [PMID] [PMCID]
10. Mihai MC, Popa MA, Suica VI, Antohe F, Jackson EK, Simionescu M, Dubey RK. Mechanism of 17β-estradiol stimulated integration of human mesenchymal stem cells in heart tissue. J Mol Cell Cardiol. 2019;133:115-24. [DOI] [PMID]
11. Mohamad HE, Askar ME, Hafez MM. Management of cardiac fibrosis in diabetic rats; the role of peroxisome proliferator activated receptor gamma (PPAR-gamma) and calcium channel blockers (CCBs). Diabetol Metab Syndr. 2011;3(1):4. [DOI] [PMID] [PMCID]
12. Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC cancer. 2017; 17(1):1-11. [DOI] [PMID] [PMCID]
13. Li Q, Sun SZ, Wang Y, Tian YJ, Liu MH. The roles of MMP-2/TIMP-2 in extracellular matrix remodelling in the hearts of STZ-induced diabetic rats. Acta Cardiol. 2007;62(5):485-91. [DOI] [PMID]
14. Sima J, Zhang SX, Shao C, Fant J, Ma JX. The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett. 2004;564(1-2):19-23. [DOI] [PMID]
15. Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, et al. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci U S A. 1998;95(10):5579-83 [DOI] [PMID] [PMCID]
16. Patel JV, Sosin M, Gunarathne A, Hussain I, Davis RC, Hughes EA, Lip GY. Elevated angiogenin levels in chronic heart failure. Ann Med. 2008;40(6):474-9. [DOI] [PMID]
17. Ghorbanalipour A A, Motamedi P, Rajabi H, & Karami H. The Effect of Endurance Training on Angiostatin and Enos Gene Expression of Cardiac Tissue in Type 2 Diabetic Male Wistar Rats. J Arak Uni Med Sci. 2019;21(7):112-22. []
18. Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296(2):H428-34. [DOI] [PMID] [PMCID]
19. Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100(3):434-43. [DOI] [PMID]
20. Ortiz R, Kluwe B, Odei JB, Echouffo Tcheugui JB, Sims M, Kalyani RR, et al. The association of morning serum cortisol with glucose metabolism and diabetes: The Jackson Heart Study. Psychoneuroendocrinology. 2019;103:25-32. [DOI] [PMID] [PMCID]
21. Sagara R, Inoue T, Sonoda N, Yano C, Motoya M, Umakoshi H, et al. Association between cortisol and left ventricular diastolic dysfunction in patients with diabetes mellitus. J Diabetes Investig. 2022;13(2):344-50 [DOI] [PubMed] [PMCID]
22. Sgrò P, Emerenziani GP, Antinozzi C, Sacchetti M, Di Luigi L. Exercise as a drug for glucose management and prevention in type 2 diabetes mellitus. Curr Opin Pharmacol. 2021;59:95-102. [DOI] [PMID]
23. Vali Zadeh S, Motamedi P, Karami H, Rajabi H. The effects of endurance training on gene expression of VEGF and VEGFR2 of cardiac tissue in Type 2 diabetic male wistar. J Arak Uni Med Sci. 2018;21(6):107-18. [Article]
24. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218-27. [DOI] [PMID]
25. Layton AT, Edwards A, Vallon V. Adaptive changes in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis. Am J Physiol Renal Physiol. 2017;313(2):F199-209. [DOI] [PMID] [PMCID]
26. Crisafulli A, Pagliaro P, Roberto S, Cugusi L, Mercuro G, Lazou A, et al. Diabetic Cardiomyopathy and Ischemic Heart Disease: Prevention and Therapy by Exercise and Conditioning. Int J Mol Sci. 2020;21(8):2896. [DOI] [PMID] [PMCID]
27. Kadoglou NPE, Vrabas IS, Sailer N, Kapelouzou A, Fotiadis G, Noussios G, et al. Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 2010;36(2):144-51. [DOI] [PMID]
28. Hordern MD, Coombes JS, Cooney LM, Jeffriess L, Prins JB, Marwick TH. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart. 2009;95(16):1343-9. [DOI] [PMID]
29. Schrauwen-Hinderling VB, Meex RC, Hesselink MK, van de Weijer T, Leiner T, Schär M, et al. Cardiac lipid content is unresponsive to a physical activity training intervention in type 2 diabetic patients, despite improved ejection fraction. Cardiovasc Diabetol. 2011;10:47. [DOI] [PMID] [PMCID]
30. Chae CH, Jung SL, An SH, Park BY, Wang SW, Cho IH, et al. Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience. 2009;164(4):1665-73. [DOI] [PMID]
31. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537-46. [PubMed]
32. Seguro C, Viana R, Lima G, Galvao L, Silva L, Jardim T, et al. Improvements in health parameters of a diabetic and hypertensive patient with only 40 minutes of exercise per week: a case study. Disabil Rehabil. 2020;42(21):3119-25. [DOI] [PMID]
33. Akerstrom T, Laub L, Vedel K, Brand CL, Pedersen BK, Lindqvist AK, et al. Increased skeletal muscle capillarization enhances insulin sensitivity. Am J Physiol Endocrinol Metab. 2014;307(12):E1105-16. [DOI] [PMID]
34. Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88(11):5444-51. [DOI] [PMID]
35. Khajehnasiri N, Dehkordi MB, Amini-Khoei H, Mohammadabadi MSM, Sadeghian R. Effect of exercise intensity and duration on the levels of stress hormones and hypothalamic-pituitary-gonadal axis in adult male rats: an experimental study. Hormones (Athens). 2021;20(3):483-90. [DOI] [PMID]
36. Estrada C, Cuenca L, Cano-Fernandez L, Gil-Martinez AL, Sanchez-Rodrigo C, González-Cuello AM, et al. Voluntary exercise reduces plasma cortisol levels and improves transitory memory impairment in young and aged Octodon degus. Behav Brain Res. 2019;373:112066. [DOI] [PMID]
37. Jang Y, Lee B, Kim EK, Shim WS, Yang YD, Kim SM. Involuntary swimming exercise in pregnant rats disturbs ERK1/2 signaling in embryonic neurons through increased cortisol in the amniotic fluid. Biochem Biophys Res Commun. 2018;495(1):1208-13. [DOI] [PMID]
38. Sajedi D, Shabani R, Elmieh A. Changes in leptin, serotonin, and cortisol after eight weeks of aerobic exercise with probiotic intake in a cuprizone-induced demyelination mouse model of multiple sclerosis. Cytokine. 2021;144:155590 [DOI] [PMID]
39. Rosmond R, Björntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247(2):188-97. [DOI] [PMID]
40. Drust B, Waterhouse J, Atkinson G, Edwards B, Reilly T. Circadian rhythms in sports performance--an update. Chronobiol Int. 2005;22(1):21-44. [DOI] [PMID]
41. Habibian M, Khosravi M. The Effect of 8 Weeks Regular Swimming Exercise on the Cardiac Levels of Matrix Mettaloproteinase-2 and Transforming Growth Factor-Β1 in Diabetic Rats. Iran J Diabetes Metab. 2016;15(2):67-74 [Article]
42. Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25(3):1106-17. [DOI] [PMID] [PMCID]
43. Farag HAM, Hosseinzadeh-Attar MJ, Muhammad BA, Esmaillzadeh A, Bilbeisi AHE. Comparative effects of vitamin D and vitamin C supplementations with and without endurance physical activity on metabolic syndrome patients: a randomized controlled trial. Diabetol Metab Syndr. 2018;10:80. [DOI] [PMID] [PMCID]
44. Taye A, Abouzied MM, Mohafez OM. Tempol ameliorates cardiac fibrosis in streptozotocin-induced diabetic rats: role of oxidative stress in diabetic cardiomyopathy. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(12):1071-80. [DOI] [PMID]
45. Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543-67. [DOI] [PMID]
46. Seyfi Askshahr F, Khajehlandi M. The Effect of Moderate-Intensity Endurance Training on the Expression Level of Angiogenesis Factors and Cardiac Oxidative Stress in Rats. J Ardabil Uni Med Sci. 2020;20(3):361-71. [DOI]
47. Oliveira V, Akamine EH, Carvalho MH, Michelini LC, Fortes ZB, Cunha TS, et al. Influence of aerobic training on the reduced vasoconstriction to angiotensin II in rats exposed to intrauterine growth restriction: possible role of oxidative stress and AT2 receptor of angiotensin II. PLoS One. 2014;9(11):e113035. [DOI] [PMID] [PMCID]
48. Ranjbar K, Rahmani-Nia F, Shahabpour E. Aerobic training and l-arginine supplementation promotes rat heart and hindleg muscles arteriogenesis after myocardial infarction. J Physiol Biochem. 2016;72(3):393-404. [DOI] [PMID]
49. Drixler TA, Rinkes IHB, Ritchie ED, Treffers FW, van Vroonhoven TJ, Gebbink MF, et al. Angiostatin inhibits pathological but not physiological retinal angiogenesis. Invest Ophthalmol Vis Sci. 2001;42(13):3325-30. [Article]
50. Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105(18):2185-91. [DOI] [PMID]
51. Montgomery A, MacLean D. The Effect of Acute Exercise on the Interaction between Doxorubicin Administration and Nitric Oxide Metabolism in Heart and Liver Cells. FASEB J. 2021;35(S1). [DOI]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution 4.0 International License. |







